
“These devices present a number of psychological risks including depression, anxiety, worsening of underlying symptoms, development of post-traumatic stress disorder, and physical risks such as pain, burns, and tissue damage.”

“These devices present a number of psychological risks including depression, anxiety, worsening of underlying symptoms, development of post-traumatic stress disorder, and physical risks such as pain, burns, and tissue damage.”
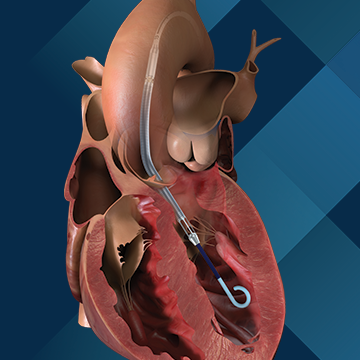
Use of the affected Impella pumps may cause serious adverse health consequences, including left ventricle perforation or free wall rupture, hypertension, lack of blood flow, and death. To date, there have been 129 reported serious injuries, including 49 reports of death.

“Artificial Intelligence and Medical Products: How CBER, CDER, CDRH, and OCP are Working Together” outlines how FDA’s medical product centers plan to address regulation of AI used in medical products and their development.

“We’ve arrived at a historically strong rule that will protect the most exposed communities from toxic air pollution while also ensuring that there will be a process that safeguards our nation’s critical supply of sterilized medical equipment.”

The new draft guidance proposes select updates to the FDA guidance document “Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions” and focuses on information FDA considers necessary to support obligations under section 524B of the FD&C Act, “Ensuring Cybersecurity of Devices.”

Post-market surveillance plays a critical role in the UK’s medical device regulatory framework. Manufacturers must prioritize establishing a comprehensive PMS system, developing clear and organized PMS plans, and promptly reporting incidents and corrective actions.

The Joint Commission and Kaiser Permanente are accepting applications now through April 30 for the 2024 Bernard J. Tyson National Award for Excellence in Pursuit of Healthcare Equity. The award recognizes major achievements in improving healthcare disparities.
Leading hospital systems are advocating for “immediate transition” to digital communication to manage supply chain disruptions and enhance patient safety.

Following a “alarming” increase in medical device submissions containing unreliable data, the FDA is reminding manufacturers and study sponsors that they are responsible for qualifying third-party test labs and closely scrutinizing all testing data.

LNE-GMED UK has been designated as a UK Approved Body to assess and certify general medical devices in accordance with Part II of the UK Medical Devices Regulations 2002. Scarlet NB UK has been designated with a focus on assessing and certifying software and AI as a medical device (AI/SaMD).