

Automation and digitization of quality processes can improve efficiency, reduce costs, and enhance product quality in manufacturing, allowing for more informed decisions that will contribute to long-term success.

Automation and digitization of quality processes can improve efficiency, reduce costs, and enhance product quality in manufacturing, allowing for more informed decisions that will contribute to long-term success.

The healthcare sector can expect some improvement in lower inflation rates and attendant pressures for the remainder of 2024. But it’s not yet out of the pandemic woods, as interest rates remain high, the shortage of professionals persists and there’s no shortage of risks. Here’s a perspective on what the year holds.

On April 17, the FDA is hosting a Virtual Public Workshop on approaches to Accreditation Scheme for Conformity Assessment Expansion.

“We’ve arrived at a historically strong rule that will protect the most exposed communities from toxic air pollution while also ensuring that there will be a process that safeguards our nation’s critical supply of sterilized medical equipment.”
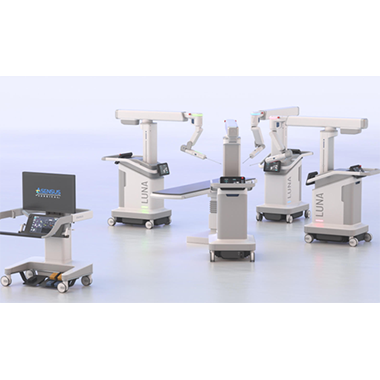
“Flex’s proven track record of delivering complex electromechanical systems across diverse industries and deep understanding of the complex requirements for medical devices will enable us to accelerate time-to-market for our LUNA platform.”

The pilot program launched by Baxter International and Northwestern Medicine in Chicago resulted in the recycling of more than 170,000 IV bags. Baxter is now working to expand the program in the Chicago area to further validate the process and economic viability of the program.

Class 1 recalls are at a 15-year high. To reduce the number of recalls and nonconformances, medtech manufacturers need quality management and traceability processes that go beyond documenting and tracking changes to effectively connect people, processes and data.

Bob Tilling, VP of Global Sales at Kallik, delves into dupe culture and its link to counterfeit drugs, and explains how developments in label management can foil illicit traders.

Stefano Vicenzetto, Design Systems Engineer at Flex, shares his insights on how Circular Economy principles can be applied within the MedTech industry to reduce environmental impact by shifting away from the linear take, make and waste approach.

The growth of the medical device industry brings increased competition and a need to find ways to become more efficient and cost-competitive, without compromising quality. In this column, Doug Donahue, Director of Foreign Direct Investment and Manufacturing for Guanajuato’s Medical Device Cluster and co-managing partner of Entrada Group, discusses the benefits, challenges and opportunities available for companies investigating Mexico as a manufacturing site.