

Outdated policies and regulations threatens to bring progress to a standstill, restrict vital telehealth access to millions of Americans, and exacerbate health inequities.

Outdated policies and regulations threatens to bring progress to a standstill, restrict vital telehealth access to millions of Americans, and exacerbate health inequities.

On the journey to full-scale production for medical devices incorporating optics, a series of steps early in the process can make all the difference for successfully launching new products and introducing next-generation upgrades. This article discusses four considerations for a successful product launch.

You’d think there’s not much to the concept of an alarm. A warning sounds in a room, or a red light flashes, and it has your attention. You know something must be wrong. But in a setting where a cacophony of alarming lights and sounds beset healthcare workers on a regular basis, these essential systems become increasingly easy to miss or ignore.

Patient-administered healthcare is one of the fastest-growing segments in the medtech industry. When the patient becomes the operator, usability requirements are vastly different than those of trained clinicians, which elevates considerations in the design process.

With the ever-increasing adoption of connected devices, the agency is emphasizing the need for effective cybersecurity.

The long-awaited part 23 of ISO 10993, the series of standards governing Biological Evaluation of Medical Devices, was published in January 2021. It was the first to introduce validated methods for in-vitro irritation tests. This article reviews the latest developments and identifies the cases in which manufacturers can avoid animal experiments.
Experts will explore how digital technologies have opened up new opportunities for patients, providers and medtech manufacturers.
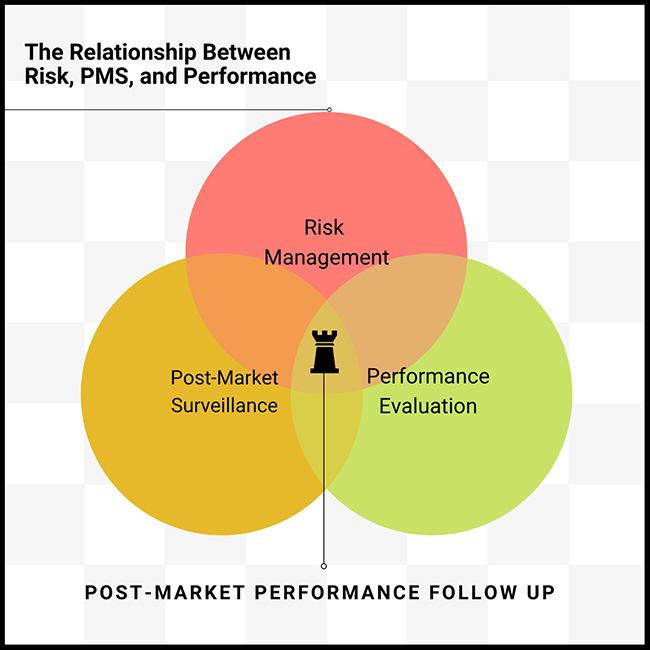
Understanding EU MDR and IVDR: The goal of EU MDR and IVDR is to ensure safety by asking manufacturers to provide evidence that their products are safe (disclosing any risks), effective (performing as expected), and state of the art (compared to industry benchmarks).

Following Karl Storz’s recall of urological endoscopes, the agency wants to make sure that healthcare providers have seen the company’s urgent labeling update involving sterilization methods.

The total $8.4 billion fiscal year 2023 budget request is 34% higher than the agency’s 2022 appropriated funding level.