

Medtech and Biotech companies that prioritize professional development and career growth can reduce turnover and attract new talent. Here is how one company is standing out in a highly competitive employment market.

Medtech and Biotech companies that prioritize professional development and career growth can reduce turnover and attract new talent. Here is how one company is standing out in a highly competitive employment market.

Gain insight into the notified body approach to assessing biological equivalence and how manufacturers can make sure their equivalence justifications pass notified body review.

Many younger employees need the interaction of in-person work. But to encourage a broader return to in-person work, organizations must make a compelling case for the benefits it brings employees. At the same time, they need to toss out the old office paradigm and transform to one that celebrates the new world of work.

There is capital sitting on the sidelines, but founders and CEOs should realize that investors are digging into their due diligence. The “wow” factor and potential size of the medtech market matter much less now than solid fundamentals.

The COVID-19 pandemic exposed the necessity of virtual care and revealed its possibility as a more efficient means of administering treatment in an overwhelmed and understaffed infrastructure. Its fast-paced adoption highlighted the need for global standards and third-party certifications.

The upcoming revision to ISO 10993-17 proposes the use of release kinetics data to support toxicological risk assessments. Manufacturers should expect that this data will be needed to help mitigate the risks identified in exhaustive extraction studies.

Leslie Trigg, CEO of Outset Medical, is the new Chair of The Medical Device Manufacturers Association (MDMA) Board of Directors.
“Addressing unmet needs across pediatric populations is critical to advancing children’s health, and we are delighted to once again work with pioneering companies that seek to bridge this care gap.”

Both legacy players and startups have an important role to play when it comes to medical innovation—they need to work together to meet the increasing demand for minimally invasive treatments, value-based care and innovative, not incremental therapies. This is a mutually beneficial relationship that is key to medical technology development.
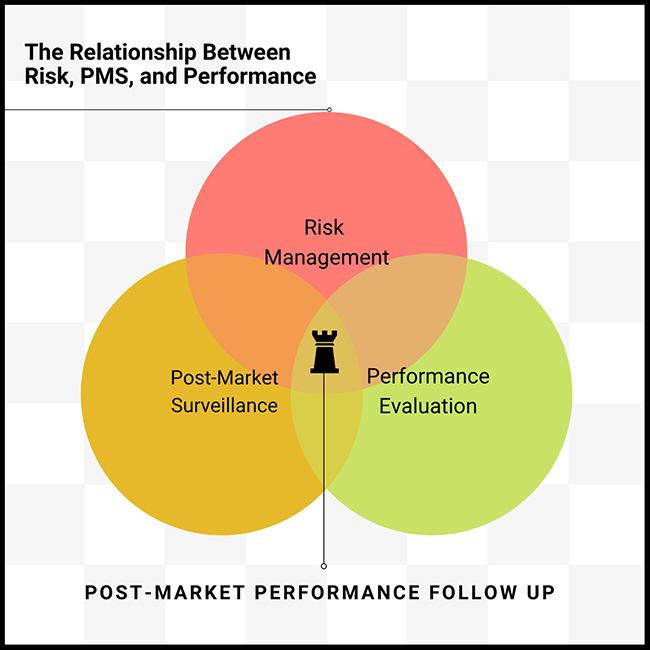
Understanding EU MDR and IVDR: The goal of EU MDR and IVDR is to ensure safety by asking manufacturers to provide evidence that their products are safe (disclosing any risks), effective (performing as expected), and state of the art (compared to industry benchmarks).