

Peter O’Blenis, CEO of Evidence Partners, discusses the growing role—and challenges –of literature reviews in the medtech market.

Peter O’Blenis, CEO of Evidence Partners, discusses the growing role—and challenges –of literature reviews in the medtech market.

Gain insight into the notified body approach to assessing biological equivalence and how manufacturers can make sure their equivalence justifications pass notified body review.

Per EU MDR regulations, any medical device on the market must be considered “state-of-the-art.” However, the term is not explicitly defined. Exploring MDR verbiage around standards harmonization, risk management and clinical data may allow a clearer understanding of regulatory expectations to emerge.

The specifications set uniform and rigorous benchmarks for tests across the EU, with the goal of clarifying the requirements for market actors and protecting EU patients.

The U.K. Medicines and Healthcare products Regulatory Agency (MHRA) has published its response to concerns raised by medical device trade associations regarding future regulation of medical devices in the United Kingdom.
The partnership brings together Evidence Partners’ literature review platform, DistillerSR, and Akra Team’s strategic regulatory services.
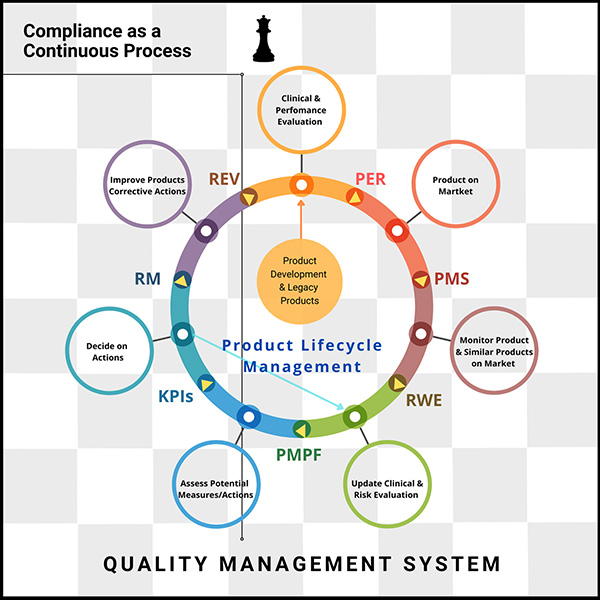
The only constant today is change itself.

The long-awaited part 23 of ISO 10993, the series of standards governing Biological Evaluation of Medical Devices, was published in January 2021. It was the first to introduce validated methods for in-vitro irritation tests. This article reviews the latest developments and identifies the cases in which manufacturers can avoid animal experiments.
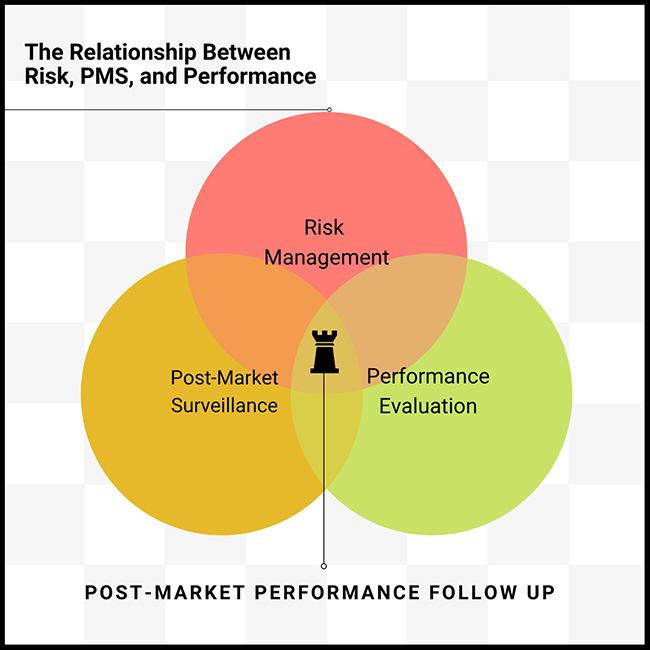
Understanding EU MDR and IVDR: The goal of EU MDR and IVDR is to ensure safety by asking manufacturers to provide evidence that their products are safe (disclosing any risks), effective (performing as expected), and state of the art (compared to industry benchmarks).

Embracing compliance is a continuous process, and investing in agile technologies that streamline workflows—especially in meeting EU MDR and IVDR requirements—is essential, says Lana Feng, Ph.D., CEO-founder of Huma.AI, a pioneer in a human-centered AI.