

Researchers have developed a new machine-learning model that can precisely make prognosis predictions for patients with osteosarcoma, based on the density of viable tumor cells post-treatment.

Researchers have developed a new machine-learning model that can precisely make prognosis predictions for patients with osteosarcoma, based on the density of viable tumor cells post-treatment.

Traditional screening tests suffer from a range of challenges. From logistical barriers to concerns regarding accuracy and reliability, achieving accurate diagnosis is frequently arduous. Imagine a revolutionary approach where early disease screening becomes as simple as collecting a breath sample. Thanks to cutting-edge sensor technology and advanced artificial intelligence, this vision is now on the brink of realization.

In February, Hologic received FDA clearance for its Genius Digital Diagnostics System, which combines advanced imaging with AI-assisted review for cervical cancer screening. We spoke with Mike Quick, who led the development of the technology, and Dr. Hans Ikenberg, director of one of the first labs to work with the system.

If adopted, companies would have additional time—from 2027 to 2029, depending on the type of device—to gain approval under EU IVDR.

Peter J. Arduini, president and CEO of GE HealthCare, began his two-year term as Board Chair last Friday. The same day AdvaMed announced its new Medical Imaging Division under the direction of Patrick Hope, former executive director of the Medical Imaging & Technology Alliance.

Companion diagnostics (CDx) play a pivotal role in personalized medicine—one of the fastest growing areas of medicine. Regulation of CDx is fairly well established in western countries, but the east is not far behind. Here we look at current and on the horizon regulatory guidances and directives for CDx manufacturers seeking to enter the Asian markets.
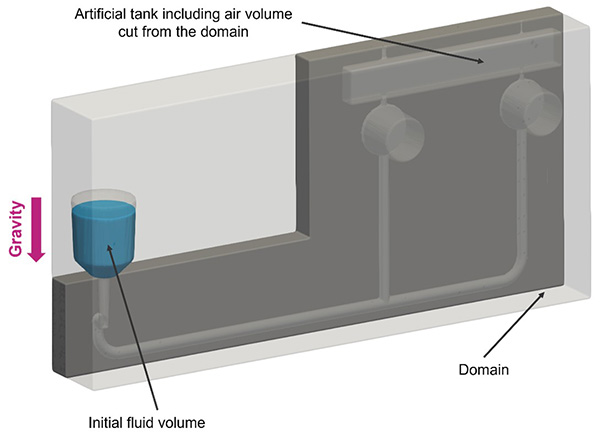
Computational simulations play a pivotal role in optimizing the design of point-of-care cassettes prior to manufacturing, shortening the development timeline and enabling swift adaptation to market demands.

Recent developments, specifically artificial intelligence and the ubiquity of smart devices, enable us to monitor cough unobtrusively and continuously for periods of time. Objective cough quantification can be combined with patients’ perceptions to better determine diagnosis, treatment response and prognosis.

Connectivity in medical devices creates new diagnostic and treatment opportunities, yet at the same time increases the risks of cyberattacks—including their consequences for patient safety and data privacy. Now the new IEC 81001-5-1 standard provides clear technical requirements for manufacturers and developers to ensure the cybersecurity of their products across their life cycle.

The specifications set uniform and rigorous benchmarks for tests across the EU, with the goal of clarifying the requirements for market actors and protecting EU patients.