Medtech Companies Improve Compliance, but Unprepared for Disaster
Medical device companies have upped their game in product security and regulatory compliance, but they might not be ready for a catastrophic event.

Medical device companies have upped their game in product security and regulatory compliance, but they might not be ready for a catastrophic event.

Regulations, cost pressures drive medical device companies to innovate.

Josh Cannon explains how technology can improve visibility throughout the supply chain.

Finding ways to better manage inventory, provide security and product integrity, and maintain continuous improvement are among the goals of logistics professionals.

Manufacturers should collaborate with a third-party logistics provider that has expertise in local in-country regulatory requirements and transportation security.
Product differentiation is important, but failing to nurture supply chain partnerships is a mistake that companies still make.
Product differentiation is important, but failing to nurture supply chain partnerships is a mistake that companies still make.
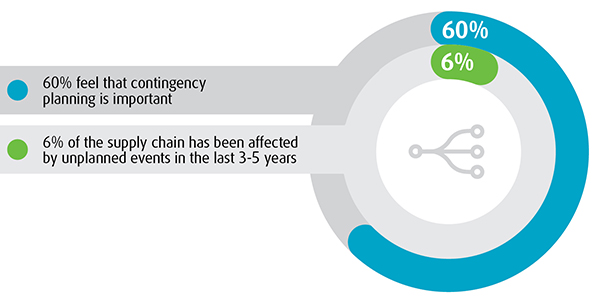
Stringent regulations, increased product protection challenges, economic pressures and contingency management have been identified as the most significant contributors to uncertainty in the healthcare supply chain.
Stringent regulations, increased product protection challenges, economic pressures and contingency management have been identified as the most significant contributors to uncertainty in the healthcare supply chain.
In order to compete globally, companies must be proactive in their business plans and anticipate the challenges they may face, concur three med-tech executives.
In order to compete globally, companies must be proactive in their business plans and anticipate the challenges they may face, concur three med-tech executives.