
Healthcare IT Solutions Expand with Rapid Digitalization Trend
Electronic health records and mHealth solutions are two of the leading development areas in the medical domain, augmenting opportunities for the healthcare IT industry.

Electronic health records and mHealth solutions are two of the leading development areas in the medical domain, augmenting opportunities for the healthcare IT industry.

Medical device manufacturers can accelerate their digital transformation priorities through lean practices to better manage supply chain risk, complexity and disruptions.

Audits for medical device companies and other organizations in the medtech space used to be primarily in person. However, the pandemic magnified flaws in the global regulatory process. A shift to remote audits may be the key to interorganizational and international regulatory harmonization moving forward.

FDA also supports the removal of the HeartWare HVAD System from the market.

The utilization of reprocessed medical devices can help cut down the increasing cost of healthcare for consumers while also tackling device shortages.

The medtech regulatory environment in Europe has entered a new era. A Europe-wide medical device regulation has come into effect, presenting both challenges and opportunities for stakeholders in the sector.

As the pace of innovation further accelerates in 2021, and the need to get new devices to market intensifies, maintaining adherence to regulatory controls is not enough.
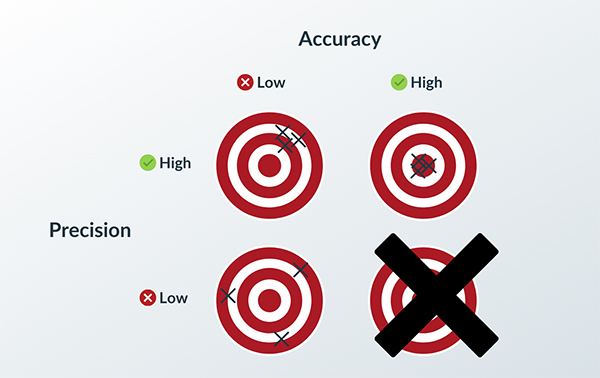
The key is to keep quality high and risk low.

Using the right strategy, remote patient monitoring turns episodic care into preventative care, potentially improving the patient experience and health outcomes.

To better understand the future of telehealth, it is important to understand what the costs look like over time and whether telehealth offers advantages in cutting healthcare costs overall.