
What’s Holding Back Use of Robotic Devices for Surgeries?
A look at the factors contributing to the slow adoption rate of these devices in the OR, along with how the industry can increase adoption.

A look at the factors contributing to the slow adoption rate of these devices in the OR, along with how the industry can increase adoption.

U.S. companies are increasingly engaging with organizations worldwide to push innovation in nanotechnology forward.

The FDA requires medical device manufacturers to demonstrate the sterility of their products. Whether a device functions in vivo or in vitro, sterility is crucial to ensuring patient safety and maximizing device functionality.

There are also reports that Elizabeth Holmes, as part of her defense, may argue ex-boyfriend and former Theranos President Sunny Balwani abused her during their relationship.

Integration with advanced AI systems and a rapidly expanding application scope will position medical robots among the key solutions for efficient, accurate and safe healthcare service delivery.

The COVID-19 pandemic has changed hospital processes and made people more aware of the need to thoroughly sterilize medical instruments between patients. This article discusses some of the changes that may occur due to lessons learned throughout the global health emergency.

The pandemic has accelerated a revolution in medtech, impacting how companies approach their business strategy as well as how healthcare systems interact with medical device companies.
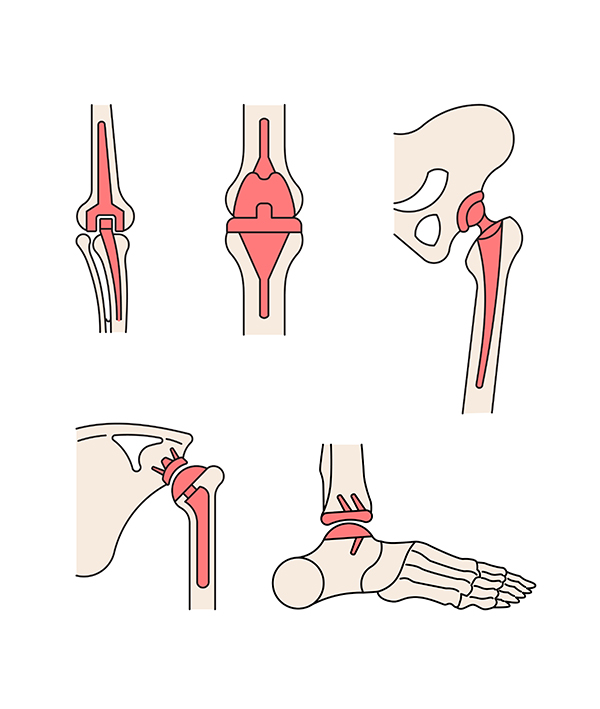
The dynamics of the joint reconstruction devices industry has strengthened with consistent technological improvements, including the adoption of 3-D printing.

In a company release, Gorsky stated the decision comes at the right time both professionally as well as personally, as he must focus on family health. Current Vice Chairman of the company’s executive committee, Joaquin Duato, will serve as new CEO, effective January 3, 2022.

As more medtech companies take advantage of the game-changing technology to innovate and advance products, they must anticipate the need to obtain trustworthy, evidence-based comprehensive data—and be prepared to do their own due diligence to verify the chain of evidence and meet increasingly stringent regulatory requirements.