
Reliable Real-time Software for Medical Devices
For reliable systems, we need a different mindset.

For reliable systems, we need a different mindset.

In addition, in workforce optimization, retention plays an equally important role as recruitment.

Manufacturers can rely upon internal human factors engineers and/or external consultants to apply HFE effectively during device development.

Why you can’t afford to overlook this important part of product development.

The unpredictability of the process can make it difficult to stay on track throughout every phase.
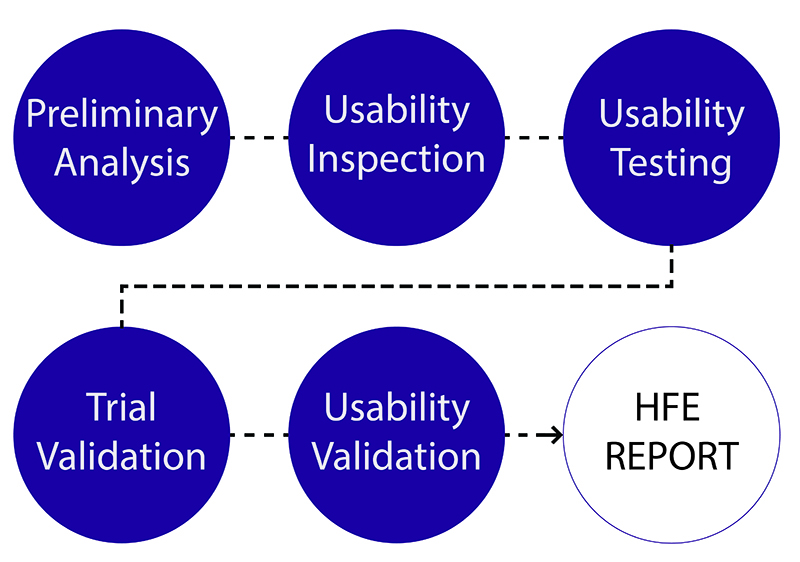
The agency calls out specific devices that should include human factors data in premarket submissions, along with recommendations for combination products.

A review of how to incorporate complaint handling and risk management into the postmarket surveillance process.

Don’t ignore the changing medtech landscape; it will continue to affect product design and manufacturing. A discussion with Kenneth Fine about design challenges.
Developing next-generation technology for medical devices is only half the battle when it comes to being first to market in today’s fast-paced global environment.
Developing next-generation technology for medical devices is only half the battle when it comes to being first to market in today’s fast-paced global environment.
From contacts and sealing, to cable assembly requirements and miniaturization, here’s a step-by-step guide for engineers to help them ask the right questions as they determine connectors they will be need for their devices.
From contacts and sealing, to cable assembly requirements and miniaturization, here’s a step-by-step guide for engineers to help them ask the right questions as they determine connectors they will be need for their devices.