Medical technology is constantly pushing boundaries, and with it comes the ever-present challenge of securing reimbursement for innovative solutions. The path to widespread adoption for any new technology is paved with regulatory hurdles, coding ambiguities, and convincing payers of its value proposition. Here, we explore the striking similarities in the reimbursement challenges faced by molecular diagnostics 20 years ago and explore the lessons that can be learned by Artificial Intelligence/ Machine Learning (AI/ML) medtech in development today. In particular, molecular diagnostics and AI/ML tools parallels include navigating evolving regulatory environments, having proprietary algorithms that represent a “black box” to stakeholders, and struggling to meet payers’ evidence thresholds.
Both molecular diagnostics and AI/ML medtech tools share an interesting conundrum. They offer a valuable step in the healthcare process, but their impact on final patient outcomes can be one or two steps removed. Despite this distance, payers still demand robust evidence of these technologies’ clinical utility, meaning they need to demonstrate a clear ability to improve overall patient health or healthcare economics, even if the technology itself doesn’t directly deliver the final outcome. This disconnect between a technology’s function and the desired long-term impact creates a hurdle for founders to overcome when convincing payers of their value proposition. By drawing parallels between these two fields, AI/ML medtech founders can navigate the complexities of reimbursement more efficiently and ensure their technologies reach the patients who need them most.
Regulatory Maze and the Coding Conundrum
Adding to the complexity is the evolving regulatory landscape. Both molecular diagnostics and AI/ML emerged during periods of regulatory flux. In addition, molecular diagnostics struggled with a lack of clear-cut coding pathways within existing American Medical Association Current Procedural Terminology (AMA CPT) codes. This ambiguity led to billing confusion and in some ways, hindered adoption. Similarly, the historical absence of dedicated codes specifically designed for AI/ML applications creates challenges for companies seeking appropriate reimbursement.
Echoes from the Past: A Landscape of Uncertainty
The rise of molecular diagnostics in the early 2000s revolutionized healthcare by enabling highly specific and sensitive testing for diseases. However, this innovation came with a price tag. Payers, accustomed to traditional laboratory diagnostic methods, were hesitant to reimburse these novel tests due to a lack of established standards for clinical validation and patient outcomes data. The technology itself was often shrouded in a veil of mystery, with “black box” algorithms that raised concerns about transparency and the decision-making processes behind the test results.
This very same scenario is playing out today with AI/ML medtech. These intelligent technologies hold immense promise for personalized medicine, early disease detection, and optimized treatment plans. Yet, payers remain cautious due to the nascent nature of the field. The lack of long-term data on AI/ML’s effectiveness and the inherent opacity of some algorithms create uncertainty around this technology category’s true value proposition.
Building the Case for Value: Beyond Novelty
Beyond the technical hurdles, convincing payers of a new technology’s value proposition remains a central challenge. Early molecular diagnostics often struggled to demonstrate a clear value advantage over traditional diagnostic methods. The focus was heavily on the novelty of the technology, rather than quantifiable benefits, such as improved patient outcomes or cost savings documented in the peer-reviewed literature. This mirrors the situation with AI/ML today. While the potential for these technologies is undeniable, companies need to move beyond simply highlighting the innovative nature of their solutions. They must present robust data that demonstrates how AI/ML can improve patient care, reduce healthcare costs, or streamline workflows for providers.
Learning from the Past: Proactive Strategies for AI/ML Founders
The Power of Pilots and Building Trust
Fortunately, history offers valuable lessons on navigating these challenges. Pilot programs proved instrumental in showcasing the effectiveness of molecular diagnostics. Real-world data collected through these pilots provided concrete evidence of the technology’s impact on patient outcomes and healthcare costs. This strategy holds immense value for AI/ML medtech companies as well. Well-designed pilot programs in collaboration with healthcare institutions can generate data that paints a clear picture of the technology’s benefits, paving the way for broader coverage from payers.
Building trust with providers is equally crucial. Just as positive experiences with molecular diagnostics from healthcare professionals influenced payer decisions, testimonials from physicians who have witnessed the positive impact of AI/ML in their practices can be a powerful tool for companies. Collaborating with relevant medical societies can further enhance trust and facilitate the development of best practices and guidelines for AI/ML use.
A Revised (and More Efficient) Roadmap
The history of molecular diagnostics offers a roadmap for AI/ML companies to navigate the complexities of reimbursement, such as:
- Advocate for Regulatory Clarity: Engage with regulatory bodies and policymakers to advocate for clear-cut regulatory frameworks that facilitate the adoption of AI/ML medtech. Additionally, advocating for clear guidelines on data security, privacy, and algorithm development can build trust with both payers and the public.
- Focus on Value Demonstration: From the outset, prioritize collecting clinical data that quantifies the impact of your AI/ML technology on patient outcomes, cost savings, or workflow improvements. Consider using cost-effectiveness models to demonstrate the technology’s long-term value. Where payers are concerned, value demonstration > value proposition.
- Explore Transparency: Demystify the “black box” by shedding light on how your AI/ML algorithms arrive at their conclusions. Ensure transparency in data collection, characterization of proprietary algorithms, and clinical decision-making processes. (Here, the American Medical Association’s CPT Appendix S may be a valuable reference point. This appendix provides language to describe the “work done by machines” in relation to the work of the physician. By leveraging the descriptors in Appendix S, AI/ML companies can position their algorithms as assistive, augmentative, or autonomous, depending on the level of physician involvement in the decision-making process.)
- Partner with Providers: Building strong relationships with healthcare institutions and providers is paramount. Understand their needs and identify workflows where AI/ML can make a significant impact. Advocacy from key opinion leaders and technology champions among healthcare professionals can be instrumental in influencing payers.
- Embrace Standardization: Promote standardization of data formats and ensure seamless interoperability with existing healthcare IT systems. Fragmented data systems can create significant challenges for billing and reimbursement. By ensuring compatibility with existing infrastructure, AI/ML companies can streamline the reimbursement process and reduce administrative burdens for providers.
A valuable framework, used extensively with molecular diagnostics, exists to guide the evaluation of new medical technologies and help address these challenges. AI/ML medtech founders can present their evidence according to analytic validity, clinical validity, and clinical utility categories. This framework provides a structured approach for assessing a technology’s performance across different dimensions.
Analytic Validity refers to the accuracy and reliability of the technology itself.
Clinical Validity focuses on whether the technology can detect or predict the intended clinical condition or outcome.
Clinical Utility is the ultimate measure of a technology’s value in real-world practice and is the category of evidence payers are most interested in. Clinical utility asks whether the technology leads to better patient outcomes, reduces healthcare costs, or improves clinical workflows.
Side by Side Comparison of Relevant Case Studies
The following table explores the aforementioned parallels between the reimbursement challenges faced by molecular diagnostics and AI/ML medtech through the lens of two real-world examples. (Table 1.) Oncotype Dx, a pioneering molecular diagnostic test for breast cancer, and Viz.ai, an AI-powered platform originally approved for stroke detection, serve as illustrative case studies. By examining the specific hurdles encountered by these technologies during their journeys to widespread adoption, we gain valuable insights into the recurring challenges faced by innovative healthcare solutions. These insights, coupled with the proactive strategies outlined earlier, can empower AI/ML companies to navigate the complexities of reimbursement and ensure their technologies reach the patients who stand to benefit the most.
Table 1. Similarities in Reimbursement Challenges Between Molecular Diagnostics and AI/ML Medtech Using Market-Available Case Studies
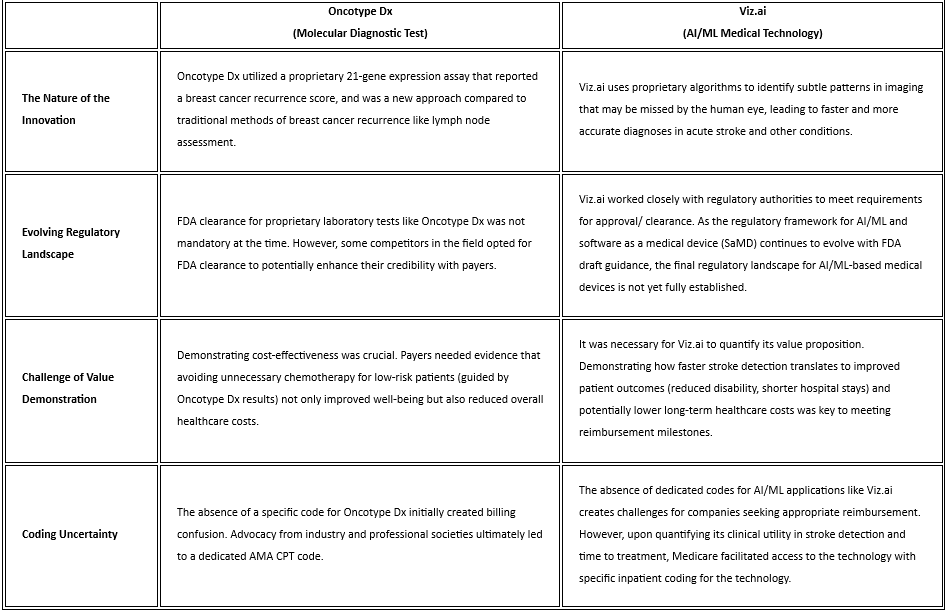
Payers have a longstanding desire for robust clinical utility data to inform their coverage decisions. In the past, this presented a significant challenge for laboratory diagnostics like Oncotype Dx. While these tests offered a novel approach to diagnosis or treatment guidance, demonstrating their long-term impact on patient outcomes and healthcare costs often proved difficult. This lack of clear clinical utility data made payers hesitant to reimburse for these new technologies. The situation is strikingly similar for AI/ML medtech today. While the potential of these technologies is clear, payers are demanding concrete evidence that translates this potential into tangible benefits. Without robust data showcasing how AI/ML improves patient outcomes, reduces resource utilization, or streamlines workflows, securing widespread reimbursement from payers remains a significant hurdle for AI/ML companies.
Conclusion: A Future of Innovation with Reimbursement in Mind
The parallels between the reimbursement challenges faced by molecular diagnostics and AI/ML medtech are undeniable. AI/ML founders can take proactive steps to navigate the complexities of securing reimbursement by understanding molecular diagnostics history. By focusing on value demonstration, embracing transparency, and fostering collaboration with both providers and policymakers, AI/ML can overcome these hurdles and become a truly transformative force in healthcare.
The journey towards widespread reimbursement for AI/ML medtech is likely to be a marathon, not a sprint. Just as molecular diagnostics required time and persistence, AI/ML companies need to be prepared for a similar trajectory. As the field continues to evolve, founders who prioritize reimbursement considerations from the outset will be best positioned to pave the way for a future where innovative AI/ML technologies are not only accessible but also seamlessly integrated into the healthcare ecosystem, ultimately improving patient care and outcomes.







