PFAS are widely used in medical technology today in devices such as catheters, stents, or implants. The reason for this lies in the properties of these synthetic per- and polyfluoroalkyl substances, of which there are estimated to be about 15,000. The carbon-fluorine bonds in PFAS are some of the strongest known in organic chemistry.
In Everyday Life
Because of these strong bonds, PFAS are chemically and thermally highly stable; they are heat-resistant, repel water, dirt, and grease, and they hardly react with other substances. These characteristics make them suitable for a wide range of applications: in everyday life, these synthetic chemicals are used to treat the surfaces of cookware and water-repellent rain jackets, and are found in fire-fighting foam, ski wax, and coated packaging. Polytetrafluoroethylene (PTFE), a special type of polymeric PFAS, is commonly known under brand names like “Teflon” and “Gore-Tex.”
Advantages for Medical Devices
While some uses of PFAS make the consumer’s life easier, in many medical fields, they are essential. Especially when artificial materials are used within the human body, they must be biocompatible and long-lasting, and should react very little (if at all) when they come into contact with blood or other bodily fluids. PFAS offer such properties, along with additional advantages, as well. For example, catheters are often lined with a PTFE which offers the benefit of minimizing friction during insertion. PFAS compounds are used in implants, pacemakers, stents, as well as in filters, tubes, and seals for dialysis. In all these cases, the stability of the molecular bonds which PFAS offer is critical.
Life-Saving — and Likely Also Hazardous to Health
These same properties — long-lasting stability and very slow degradation — are precisely what make PFAS problematic for both the environment and health. During production and disposal, as well as during use — for example, in the case of fire-fighting foam — PFAS can enter water and soil, where they accumulate. PFAS can reach water and soil, where they are persistent, and from which point they can further enter the food chain. PFAS have been detected in plants, animals and drinking water, and can thereby end up in the human body.
Though not yet conclusively proven by science for all PFAS, studies indicate that such accumulation in the body can pose health risks. Certain PFAS are very likely to negatively impact fertility, the human hormonal system, and the development of unborn life. Furthermore, some PFAS are associated with liver and kidney damage, and there is suspicion that several of these substances may be carcinogenic. However, there is currently no evidence that medical devices release significant amounts of PFAS during their use — but they may do so during production and disposal.
Initial Restrictions
These risks to health and the environment are now being discussed worldwide, with conflicting interests on both sides: the advantages of stable chemicals, especially for medical devices, versus their disadvantages for health and the environment. Some actions have already been taken. Since 2020, the use of perfluorooctanoic acid (PFOA) has been banned in the European Union, and perfluorooctanesulfonic acid (PFOS) has been heavily restricted. In the United States, the Environmental Protection Agency (EPA) has set limits on certain PFAS in drinking water.
EU Initiative Against PFAS
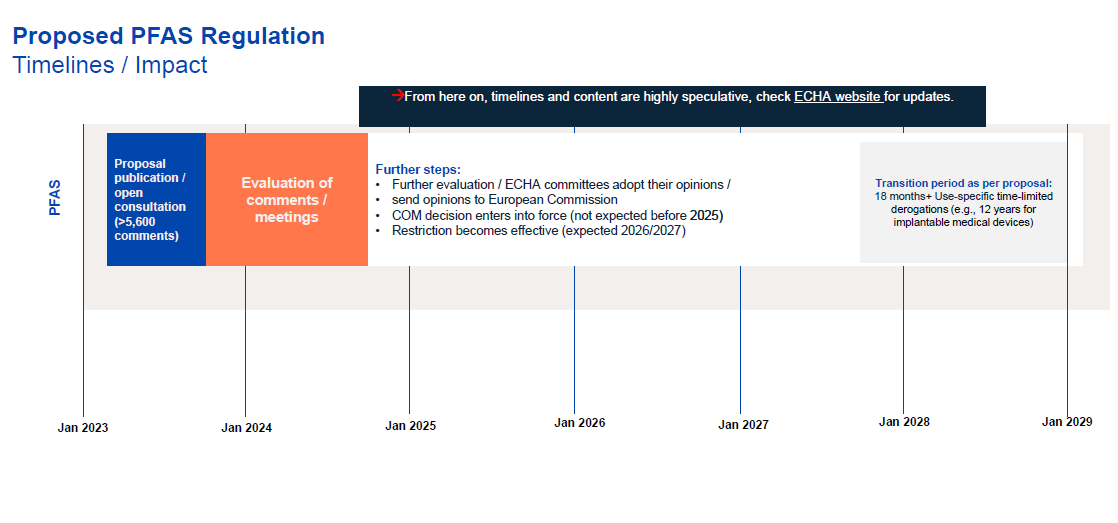
In the spring of 2023, five countries submitted a proposal regarding PFAS to the European Union: the Netherlands, Germany, Denmark, Norway, and Sweden. They have drafted a proposal to permanently ban PFAS with a general transition period of 18 months — and with use-specific derogations allowing longer transition periods. The proposal was open to the public for comments. When the comments have been discussed in committees and final conclusions have been prepared, the European Commission, along with member states and the EU Parliament, must decide on these, with a decision expected no earlier than 2025. At that time, concrete details on the future regulation of PFAS production and use will be outlined, along with any potential transition periods for a ban. It is anticipated that the earliest bans could start in 2026 or 2027. However, it is already clear that, due to the lack of alternatives, certain applications and products would require longer transition periods, currently proposed to be five and twelve years.
Search for Alternatives
The prohibitions and deadlines depend largely on available alternative chemicals that could replace PFAS. In some areas, this should be feasible in the near term, such as replacing PFAS-coated pans with cast iron or ceramic-coated options. In other cases, however — especially in the medical field — it is still unclear how to replace these stable chemicals.
This will require intensive research and development in the future to develop alternative substances and processes. These, in turn, must be tested and approved, which is known to be time-consuming in the medical field. The availability and safety of alternative substances will thus be crucial in ensuring the supply of medical devices. It is worth noting that, regardless of whether the ban is fully implemented, delayed, or only partially enacted, medical technology manufacturers using PFAS are already facing a need for action.
Researchers are already active in developing alternatives, and many PFAS producers are phasing out the production of these controversial chemicals. Therefore, it is possible that a supplier may cease offering PFAS regardless of regulatory bans. Medical device manufacturers should prepare for this possibility now by reviewing their supply chains and products to identify which substances may become problematic in the future — whether due to a ban or supply shortages.
Making the Shift
Addressing the PFAS issue is already essential for medical device manufacturers to prepare for the development and use of alternatives. Companies like TÜV SÜD offer a variety of support options. In addition to comprehensive information, they can help identify PFAS in a company’s production chain. Once potential replacement products are identified, TÜV SÜD’s laboratories can test them, examining both their chemical properties and biocompatibility, and validating packaging systems, as well.
Despite the indeterminate decision on the EU’s PFAS ban, one thing remains certain: with the impending shift away from the use of PFAS, suppliers and manufacturers should start planning as early as possible to address how they will go about the change.
MedTech Intelligence Content Partner
The author is employed by or otherwise directly associated with this Partner.