

With the goal of acting as a single agency, FDA’s OCP has several activities planned for this year.

With the goal of acting as a single agency, FDA’s OCP has several activities planned for this year.
Learn about the current guidance for Cybersecurity in the medical industry and strategies for how you can demonstrate to regulators, purchasers, and users that you’ve addressed these threats.

The bill has received bipartisan support for removing barriers and accelerating innovation in medical devices, drugs and biologics.
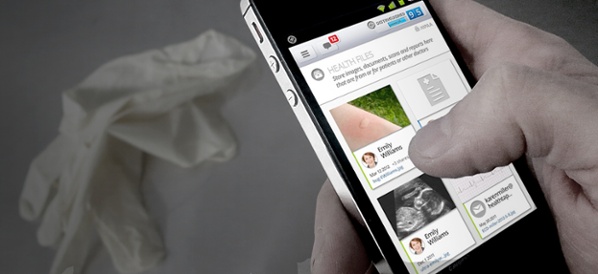
As wearable technology within the medical device industry heats up, FDA makes some clarifications on which products fall into general wellness and low-risk categories.

What are the challenges faced by OCP?

The comprehensive approach to human factors considers how a product operates alone, interoperability in larger settings, and data management.
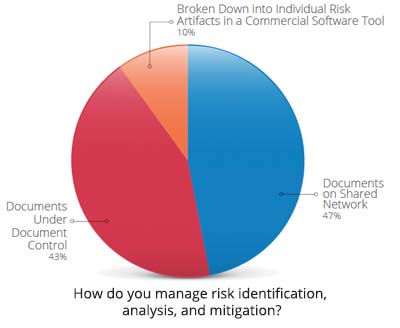
With the increased complexity of devices, a streamlined approach to managing product development risks and documenting compliance is challenging but perhaps more important than ever.
There are several steps you can take to protect your IP and avoid medical device piracy in China.
The creation of the Office of Combination Products more than a decade ago may have been a big step forward, but frustrations surrounding policy-making and coordination between CDRH, CDER and CBER remain.
REACH, which stands for the Regulation, Evaluation, Authorization and Restriction of Chemicals, is a standard that was established by the European Union (EU) in 2007 but does not go into effect until 2018. Simply put, REACH immediately seeks to “limit or prohibit the use of toxic substances in products.” According to an article written by…