
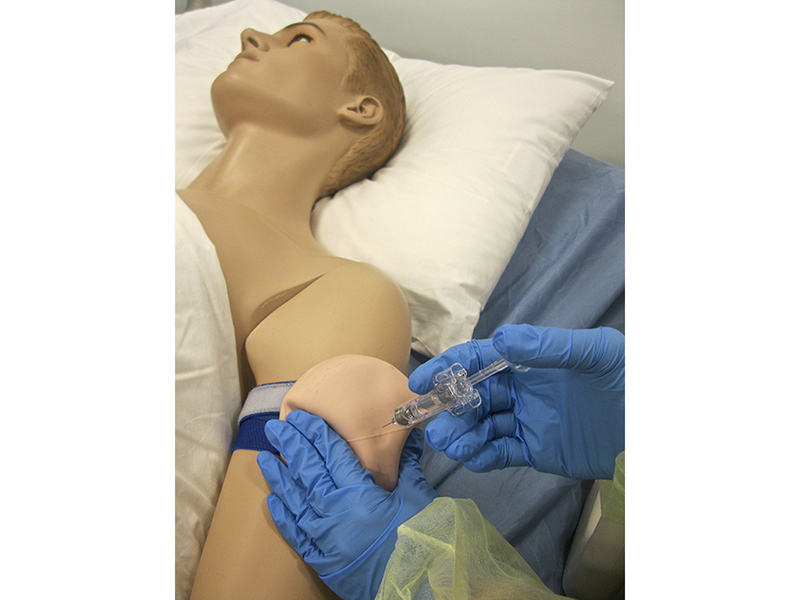
Augmented Reality Set to Revolutionize Global Medical Education Methodologies
Transformational technology is delivering effective and efficient training with precision.

Transformational technology is delivering effective and efficient training with precision.

The agency wants device manufacturers to participate in the MDSAP Pilot.
Conducting usability tests will help manufacturers reduce the risk of harmful use errors and enhance device effectiveness.

As the agency continues to try to do more with fewer resources, taking additional risks is imperative to move forward.
Training is one of the areas that result in many non-conformances being issued during quality system audits.
Training is one of the areas that result in many non-conformances being issued during quality system audits.

The Quality System Regulation (QSR), for production and process controls, (21 CFR, Part 820 – Subpart G, Section 820.70) is in the opinion of Dr. D one of the more salient requirements of the regulation.

FDA is really taking medical device manufacturers to task in regards to purchasing controls and the overall management of their suppliers. According to Kimberly Trautman, FDA’s current Good Manufacturing Practices (cGMP) and Quality System Regulations (QSR) expert, suppliers providing non-conforming material are directly related to an increase in medical device recalls; which increases the need for effective quality processes to mitigate risk. As the medical device industry continues to grow in leaps and…

The control of documentation is one the fundamental cornerstones supporting the foundation needed for an effective quality system, whether compliance is to EN ISO 13485, EN ISO 9000, the Medical Device Directive, or in the case of this series of Devine Guidance articles, the Quality System Regulation (QSR).

In this fourth and final foray into Subpart C – Design Controls, Dr. D will review the last three subsections; (h) design transfer, (i) design changes, and (j) the design history file (DHF), located within section 820.30. These final three elements of design control are just as important as the previous subsections dissected as part of Devine Guidance.

The proverbial rubber meets the road when the actual execution of test protocols commences. In this edition of Devine Guidance , Dr. D will continue with his dissection of 21 CFR, Part 820; Section 820.30, subsection f (design verification) and subsection g (design validation).