
Medical Device Manufacturing: Five Challenges in Maintaining Compliance
The industry struggles with various unknowns that impact efficiency, quality, and as a result, finances. This article reviews some of these challenges and how to overcome them.

The industry struggles with various unknowns that impact efficiency, quality, and as a result, finances. This article reviews some of these challenges and how to overcome them.

Achieving EU MDR compliance is considerably challenging for many businesses, which is why they must be discerning when it comes to selecting suitable partners.

Although the full impact of COVID-19 is uncertain, one sure thing is that industry continues to crave information to help them navigate EU MDR.

Managing how a material can affect a device’s success starts with a strong, collaborative relationship with your materials supplier, which can help prevent a myriad of issues throughout the development of medical devices.

The medical device industry is pressured to aid those stricken by the pandemic, while at the same time working to mitigate increased risks usually associated with hurried manufacturing and quality control procedures.

Launched by AdvaMed, the platform was developed in collaboration with partners that include the Aerospace Industries Association and Google.

COVID-19 requires us to rethink our current device disinfection procedure.
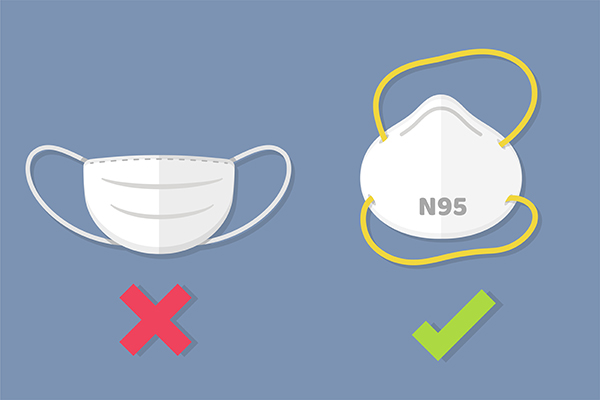
As the saying goes, if it looks too good to be true, it probably is.

For too long this industry has lagged the pharma sector in terms of using data analytics to predict market trends. It’s time to change that.

There are several critical questions that your internal team should be ready to address when preparing product portfolios and seeking out a CRO.