
Three Things You Need to Know About FDA QSR and ISO 13485 Harmonization
New changes mean new challenges, but global harmonization could make things easier for device manufacturers in the long run.

New changes mean new challenges, but global harmonization could make things easier for device manufacturers in the long run.

EU regulations require manufacturers of medical devices and systems prove the single-fault safety of their products. However, it is not clearly defined in detail how to comply with these requirements. This article explains which technical and legal requirements apply and which aspects should be considered during development.

Intensive chemistry requirements are creating massive data sets of chemicals, all of which need to be assessed for biological safety. This deluge of data is making equivocal results far more common than ever before. Fortunately, manufacturers can take steps to mitigate risk and support the safety of their products.

It’s important to draw on experience from other sectors.

To avoid delays in timelines, companies should develop IFU cleaning instructions with the worst-case clinical use and contamination method in mind.

Smart process automation promises to cut complexity and transform product traceability in the medical device industry. However, its potential relies 100% cent on having good, reliable data on which to draw.
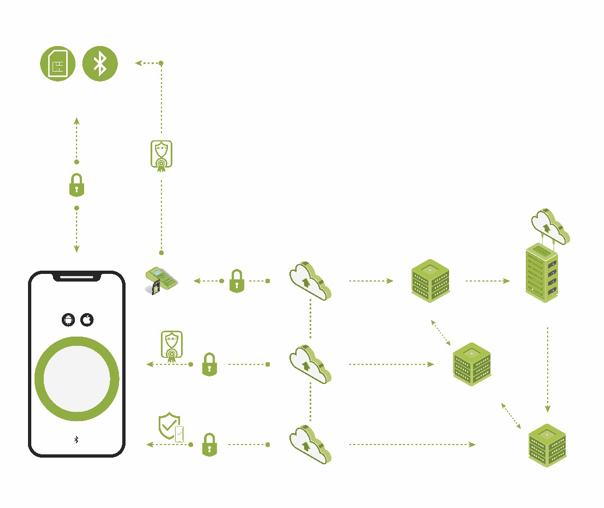
The more devices are connected, the more that targets are present for remote attackers.

Drop the checkbox mentality and embrace accountability and understanding.
Wireless medical applications have the potential to improve the quality of care, efficiency and patient comfort as well as reduce medical errors.

As standards around interoperability emerge, now may be the time to look at the benefits of connected technologies.