
Medical Device Manufacturing: Five Challenges in Maintaining Compliance
The industry struggles with various unknowns that impact efficiency, quality, and as a result, finances. This article reviews some of these challenges and how to overcome them.

The industry struggles with various unknowns that impact efficiency, quality, and as a result, finances. This article reviews some of these challenges and how to overcome them.
The key is to keep quality high and risk low.

It’s time to take stock on what temporary measures need to be made permanent to grow as an industry.

Labeling is as critical as quality for medical device products. If a manufacturer can’t shift labeling to meet production in an agile manner, their products may not reach their destination.

CAPA consistently remains a top reason for FDA’s 483 observations, usually due to inadequate processes or inconsistent adherence to regulatory requirements.

Know how IQ, OQ and PQ—the three essential elements of a Quality Assurance System—govern the medtech sector.

Furry critters have no place in a medical device manufacturing facility.
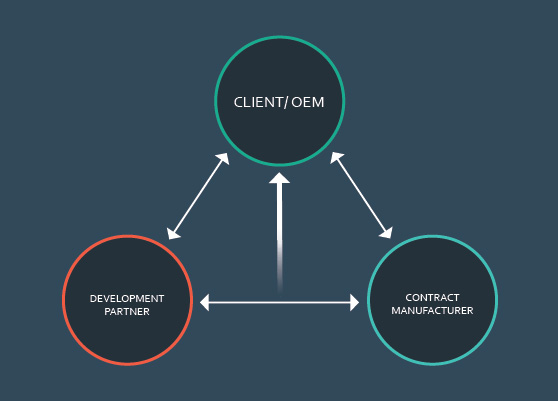
As medical device companies move technology concepts into the commercialization stage, collaborating with outside partners is crucial.

Manufacturers that make custom devices must move beyond conventional engineering practices to an automated and collaborative engineer-to-order process.

In this week’s edition of Devine Guidance , Dr. D will complete (it’s about time!) the review of section 820.70 of the Quality System Regulation (QSR), and focus on subsection (h) manufacturing material, and (i) automated processes.