
Designing the Worst-Case Test to Get Best Case Results
To avoid delays in timelines, companies should develop IFU cleaning instructions with the worst-case clinical use and contamination method in mind.

To avoid delays in timelines, companies should develop IFU cleaning instructions with the worst-case clinical use and contamination method in mind.

Although medical device manufacturers have more time to prepare due to the delayed EU MDR deadline, this shouldn’t distract from the extensive documentation they must compile in the meantime to prove their devices are compliant.

As the proliferation of connected and complex medical devices grows, healthcare providers are more susceptible to cyberattacks.

Digital technologies offer more efficient and patient-friendly distribution of the up-to-date manufacturing details and safety advice mandated by regulators. But many firms in the medtech sector are not maximizing the opportunity to deliver regulated product information digitally.

COVID-19 requires us to rethink our current device disinfection procedure.

Taking a bare-minimum approach to meeting the eIFU requirements of EU MDR could mean missing an opportunity to improve transparency in healthcare. Here’s how to use eIFU to provide stakeholders with greater confidence and clarity in medical devices.

During an outbreak, it is critical to treat reusable medical devices as potential sources of transmission.

This online series of workshops will arm you with the information you need to overcome challenges and achieve compliance with EU MDR.
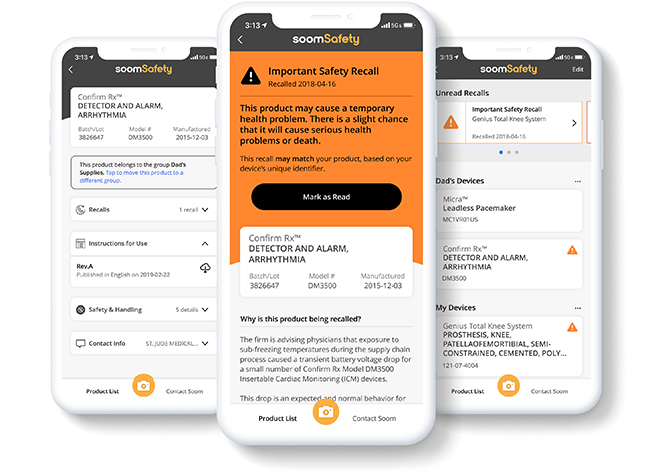
By simply scanning a barcode, SoomSafety gives users access to important product instructions, safety and recall information.

The regulation is bringing digital enablement to the forefront, requiring electronic instructions for use.