How the COVID-19 Response Is Revealing Potential of IDMP
The pandemic is helping realize the potential of ISO IDMP data standards in relation to adverse event reporting, electronic prescribing and medicines control in the supply chain.
The pandemic is helping realize the potential of ISO IDMP data standards in relation to adverse event reporting, electronic prescribing and medicines control in the supply chain.

While connected capabilities and wireless technology certainly lead to greater patient care, they also expose devices to greater cybersecurity risks.

As the proliferation of connected and complex medical devices grows, healthcare providers are more susceptible to cyberattacks.

Vulnerability sharing arms stakeholders with the information they need to assess devices, minimize cybersecurity risks and proactively mitigate emerging risks to prevent exploitation.

Make sure your 510(k) submissions pass with flying colors. Explore the latest regulations impacting bacterial endotoxin testing.
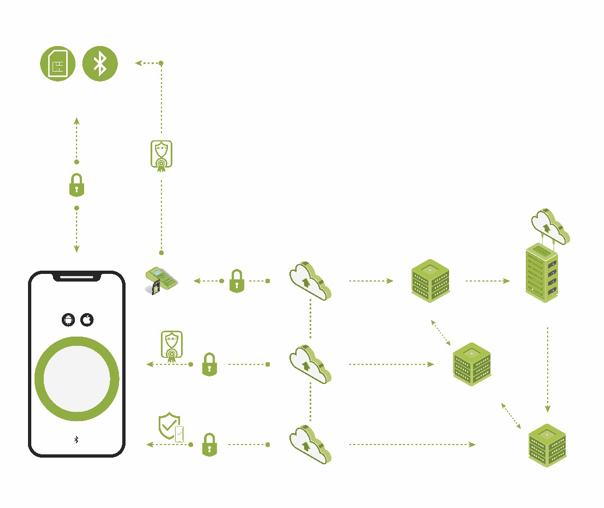
The more devices are connected, the more that targets are present for remote attackers.

This new pathway is a considerable change for the medical device industry.

The agency’s intent is to decrease regulatory burden while promoting patient access to products.

How in touch are you with current MDR guidelines?

It is surprising how little many people in the medical device industry know about freely-, widely-available resources. Often, many do not know how easy it is to find the regulations and the preambles, or about the existence of Food and Drug Administration mailing lists, phone directories and databases. MedTech Intelligence presents a basic list of non-commercial regulatory reference links, most from FDA itself. Undoubtedly, we have missed some. Send us your suggestions to make this a ‘living, growing’ list.