
Companies Shifting Focus to Better COVID-19 Testing
In order to meet the worldwide need for faster and affordable testing processes, companies must shift goals and apply resources to ending this pandemic.

In order to meet the worldwide need for faster and affordable testing processes, companies must shift goals and apply resources to ending this pandemic.

In general, the Asian markets have controlled the COVID-19 virus successfully outside of China, but its effect has still led to new developments and trends.

Across our industry, IoMT will enable us to conduct research in ways that would never previously have been possible.

AdvaMed’s Diagnostic Supply Registry is tracking the progress of molecular diagnostic tests to help support state and federal governments.

The authorization is a step toward broad screening that will help reopen schools and workplaces in America, says FDA Commissioner Stephen Hahn, M.D.

With everything to gain, telehealth may lead to a new standard in health/wellness treatment while at the same time unifying the entire healthcare ecosystem.

Companies developing technologies that integrate AI need to consider regulatory concerns, community demographics, fitting into existing workflows, technical proficiency of both the hospital personnel and consumers.
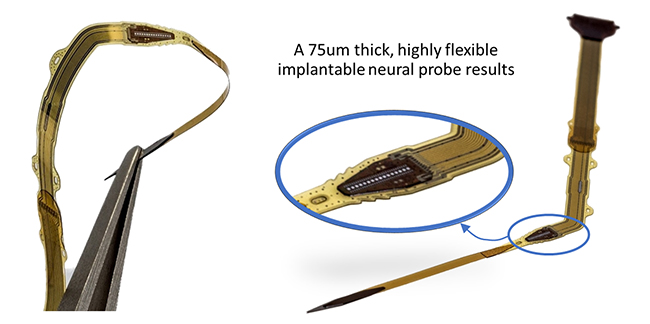
When a device is implanted in a human body, there is always a distinct reaction of the body’s immune system, often resulting in a thick layer of scar tissue surrounding the implant. With device miniaturization and by providing a ‘biomimetic’ device encapsulation, this body reaction will be reduced.

Operating with an antiquated QMS raises your risk. Here’s what to consider when modernizing your system.

The medical device industry is pressured to aid those stricken by the pandemic, while at the same time working to mitigate increased risks usually associated with hurried manufacturing and quality control procedures.