
Artificial Intelligence Applications, Regulatory Approvals on Rise in Asia
The Chinese government is investing heavily in the development of new technologies that leverage AI. This includes solutions to address the COVID-19 outbreak.

The Chinese government is investing heavily in the development of new technologies that leverage AI. This includes solutions to address the COVID-19 outbreak.

AdvaMed has reported that its member companies have provided $26.8 million in medical supplies, along with $4.1 million in cash donations.

FDA, CDC and other agencies are trying to foster the development of diagnostics, therapeutics and vaccines that help address the outbreak.

A discussion of the changing medtech markets and how companies can remain cognizant of potentially disruptive changes in their business and industry for future success in their product portfolios, services and relationships.
It’s time for medtech design engineers to take a page from the enterprise security playbook.

The agency’s move is a win for early screening of a rare genetic disorder, which leads to progressive muscle deterioration.

Medical device companies will be driven to evolve their business models to a more patient/consumer-centric approach.

The transition to digital in healthcare is happening at a fast pace.
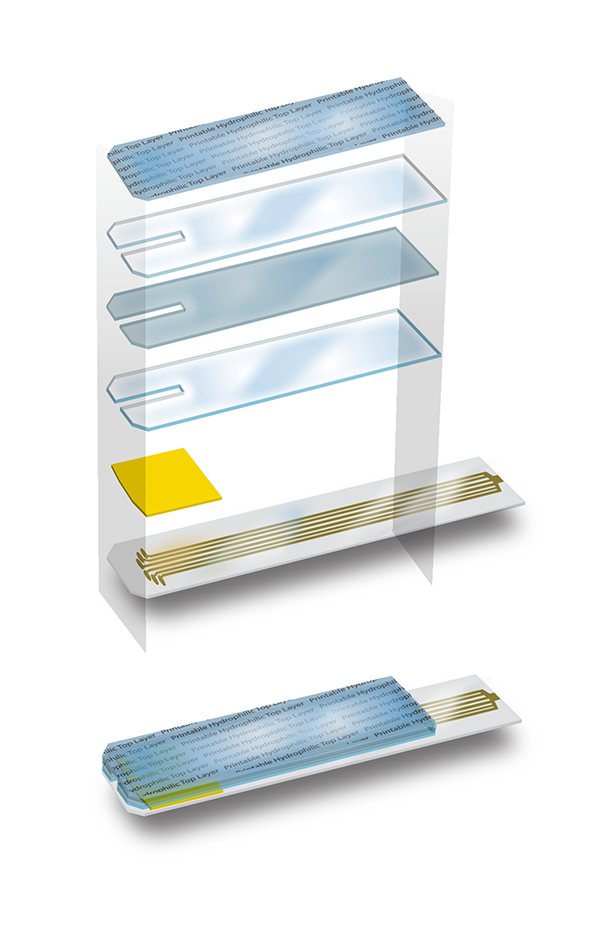
A brief look at the science behind the test strip.

Research and development activities to find potential treatments using next generation sequencing for various fatal conditions including melanoma, epilepsy, antibody deficiency, and other diseases have gained traction. In April, FDA finalized two guidance documents to speed up development of NGS-based tests.