
What Are the Biggest Changes under EU MDR?
With only one month until the compliance deadline, many medical device companies still have much to prepare in order to meet the requirements.

With only one month until the compliance deadline, many medical device companies still have much to prepare in order to meet the requirements.

Anyone can read the regulation. The challenge is in how to apply it to your company’s structure and product line.

Software continues to be a key factor in recalls, and this year the end of some EUAs may also contribute to an increase.

Labeling is as critical as quality for medical device products. If a manufacturer can’t shift labeling to meet production in an agile manner, their products may not reach their destination.

Regardless of the reason for disposing of a medical device or other electronic equipment, the product must be destroyed in a manner in which it can never be reused or identified as coming from your organization. In addition, the resulting materials from the destruction process be disposed of in an environmentally appropriate and regulatory compliant manner.

For pharmaceutical and medical device manufacturers, computerized systems validation is vital. The following are three areas businesses must fine tune to ensure computerized labeling systems meet the stipulations of the new GxP regulations.
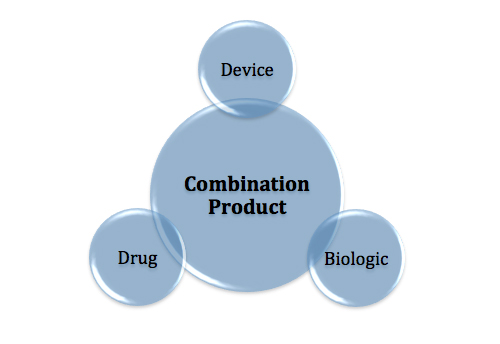
Understanding the GMP requirements of each component is critical to product and compliance success.

Moving too quickly in the product development process can lead to mistakes.
The pandemic is helping realize the potential of ISO IDMP data standards in relation to adverse event reporting, electronic prescribing and medicines control in the supply chain.

Medical device security needs to address the cyber-physical threats, not just patient health information risk.