Mike Baird

…does not expect family caregivers to provide any supplemental care. Common primary diagnoses include mild infections and chronic diseases, such as respiratory infection and heart failure.” Download the full brief…
…does not expect family caregivers to provide any supplemental care. Common primary diagnoses include mild infections and chronic diseases, such as respiratory infection and heart failure.” Download the full brief…

…for public comment. The FDA encourages stakeholders to provide comments in the Federal Register under docket number FDA-2024-N-3616. The last day to submit comments is October 4, 2024. Submit Comments…
…for public comment. The FDA encourages stakeholders to provide comments in the Federal Register under docket number FDA-2024-N-3616. The last day to submit comments is October 4, 2024. Submit Comments…

…device combinations; UK MHRA on international recognition of devices Corporate: Commission updates Accounting Directive Size Criteria; French law to increase financing of businesses Competition: Active enforcement of competition law Compliance:…
…device combinations; UK MHRA on international recognition of devices Corporate: Commission updates Accounting Directive Size Criteria; French law to increase financing of businesses Competition: Active enforcement of competition law Compliance:…

…through comprehensive risk management practices, and two key frameworks play a crucial role: ISO 14971:2019 and the FDA Quality Management System Regulation (QMSR). This essay explores their individual contributions and…
…through comprehensive risk management practices, and two key frameworks play a crucial role: ISO 14971:2019 and the FDA Quality Management System Regulation (QMSR). This essay explores their individual contributions and…
…Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced a comprehensive framework for conformity assessments, which are examinations of a manufacturer’s technical documentation and quality management system. Comprehending…
…Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced a comprehensive framework for conformity assessments, which are examinations of a manufacturer’s technical documentation and quality management system. Comprehending…
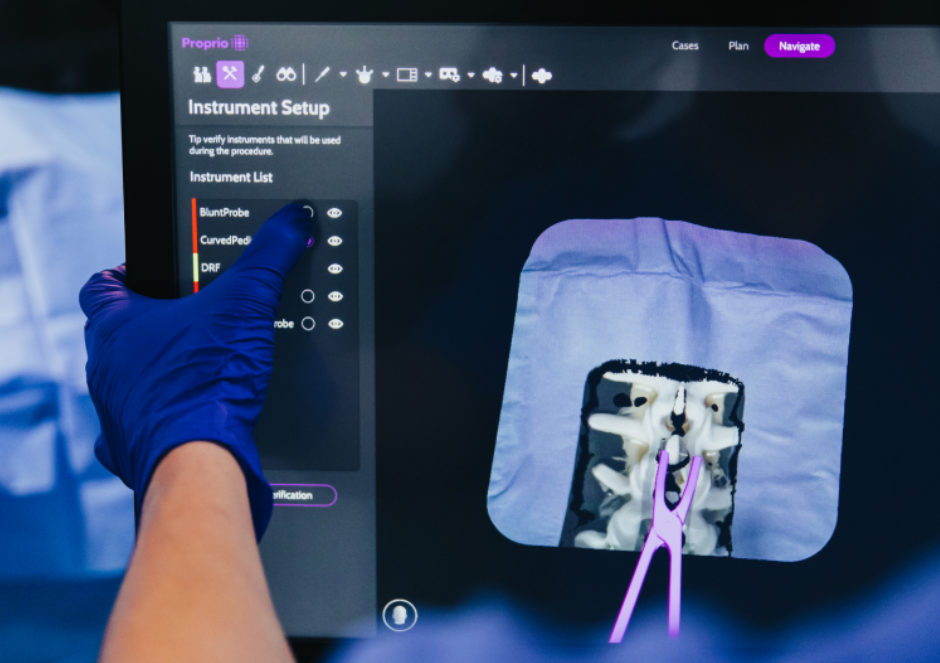
…such as advanced computational imaging techniques to address this escalating issue. Advanced Computational Visualization Techniques The complexity of adult spinal deformity cases points to the importance of precision in appropriate…
…such as advanced computational imaging techniques to address this escalating issue. Advanced Computational Visualization Techniques The complexity of adult spinal deformity cases points to the importance of precision in appropriate…