Wayne Brinster

…landscape and focus on competitor devices. View content such as research literature, clinical studies, websites, brochures, sell-sheets, instructions for use, and labeling to identify potential predicate device(s) with the same…
…landscape and focus on competitor devices. View content such as research literature, clinical studies, websites, brochures, sell-sheets, instructions for use, and labeling to identify potential predicate device(s) with the same…

…judged by a combination of clinical and data science subject matter experts, and were evaluated on several aspects, including: Innovation in clinical predictors Completeness of data science approach Statistical metrics…
…judged by a combination of clinical and data science subject matter experts, and were evaluated on several aspects, including: Innovation in clinical predictors Completeness of data science approach Statistical metrics…

…“This new rechargeable neurostimulator technology provides me with insights into my patients’ symptoms and can capture data even when they’re outside of the clinic,” said Eleni Okeanis Vaou, M.D., FAAN,…
…“This new rechargeable neurostimulator technology provides me with insights into my patients’ symptoms and can capture data even when they’re outside of the clinic,” said Eleni Okeanis Vaou, M.D., FAAN,…

Sudhir Kesavan is the new Chief Operating Officer (COO) of CitiusTech, a provider of healthcare technology services, solutions and platforms. In his new role, he will assume responsibility for leading…
Sudhir Kesavan is the new Chief Operating Officer (COO) of CitiusTech, a provider of healthcare technology services, solutions and platforms. In his new role, he will assume responsibility for leading…

…new regulations via a series of new Statutory Instruments (SIs). The agency will put priority measures to protect patient safety into place this year, with core elements of the new…
…new regulations via a series of new Statutory Instruments (SIs). The agency will put priority measures to protect patient safety into place this year, with core elements of the new…
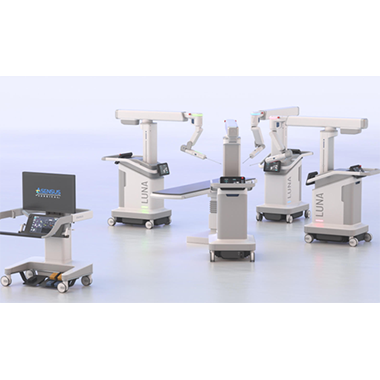
“Flex’s proven track record of delivering complex electromechanical systems across diverse industries and deep understanding of the complex requirements for medical devices will enable us to accelerate time-to-market for our…
“Flex’s proven track record of delivering complex electromechanical systems across diverse industries and deep understanding of the complex requirements for medical devices will enable us to accelerate time-to-market for our…

…June 3-5, 2024, Washington, DC, offers education and discussion for the Medtech community as it works to develop processes, procedures and solutions to streamline global and product lifecycle regulatory compliance….
…June 3-5, 2024, Washington, DC, offers education and discussion for the Medtech community as it works to develop processes, procedures and solutions to streamline global and product lifecycle regulatory compliance….

…will be accepted until Feb. 2, 2024, and will be posted to www.regulations.gov. The information collected will inform the drafting of guidance that NIST will release for public comment. …
…will be accepted until Feb. 2, 2024, and will be posted to www.regulations.gov. The information collected will inform the drafting of guidance that NIST will release for public comment. …

Baxter International announced that it has completed the first phase of its intravenous (IV) bag recycling program pilot and is now expanding the pilot program. Launched in conjunction with Northwestern…
Baxter International announced that it has completed the first phase of its intravenous (IV) bag recycling program pilot and is now expanding the pilot program. Launched in conjunction with Northwestern…