Fueled by smaller and more flexible electronics, the global market for wearable medical devices is expected to hit $7.8 billion by 2020, according to a report just released by Modor Intelligence, LLP. Wearable electronics and medical technologies are coming together at an “interesting friction point”, said Johan Kortas, senior business development manager at the Holst Centre based in Eindhoven, The Netherlands, and imec (Flanders, Belgium). The “younger” elderly population is a large technology driver in this segment, as designers try to develop products that encourage preventive care. Part of the design strategy now takes into consideration the user’s perspective first, but the technical goal remains the same—to develop better sensing and algorithms with lower power. With this objective in mind, several challenges remain in developing wearable sensors: Enabling integration with systems, providing ultra-low power and a low cost, all while offering a personalized experience for patients, and most importantly, ensuring privacy and protecting information sharing, according to Kortas.
During a recent visit to the Holst Centre, MedTech Intelligence was given a preview of wearable medical solutions that aim to address these challenges in technology development. With a goal of positioning itself between universities and industry, the Holst Centre has an impressive list of industrial partners (more than 40 total) that include Samsung, Sony, Panasonic, Evonik, Henkel, Microsemi, BASF, Under Armour, CardioNet, and Philips. These companies are collaborating to push technologies from development into testing, clinical research, and ultimately, the $3.3 billion wearable medical device market.

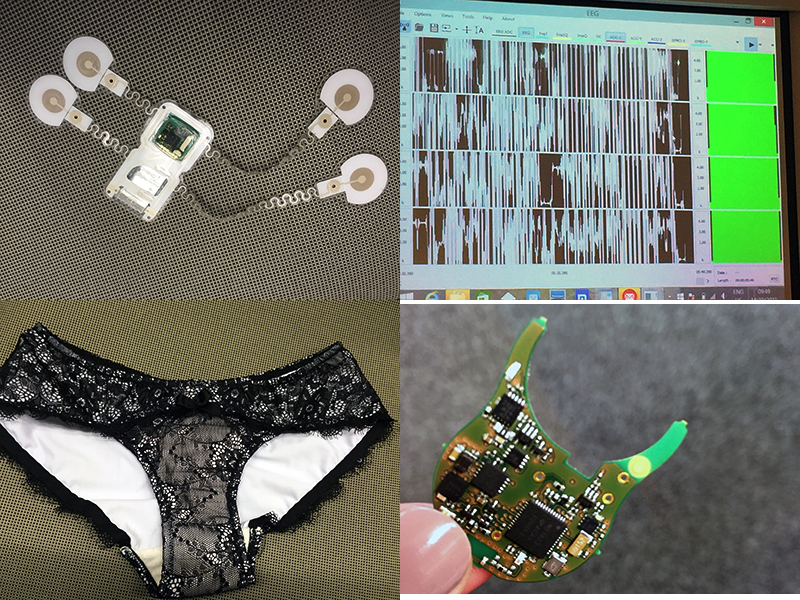
Advanced brain monitoring takes the form of a wireless electroencephalogram (EEG) headset that integrates fewer low-power electronics and measures brain activity in real time. The challenge with current EEG technologies is that they need to have good contact with the skin and thus can be uncomfortable. The device developed at the Holst Center has a more flexible design, consisting of silver/silver chloride dry electrodes that have small pins to penetrate the hair. It takes 15-30 seconds to get a signal. Addressing motion artifacts, the placement of the sensor next to electrodes improves signals and helps reduce the noise that can occur between the electrodes and the patient’s head.
The newly established LifeSense (established in July) is marrying the taboo with an unmet need via the introduction of its Carin system. On the surface, it appears to be a modern ladies panty, but much more has gone into the design of the product. According to company Founder and CEO Valer Pop, its sensor technology is one of the thinnest in the world. The sensor unit, which includes a rechargeable battery that lasts about 10 days, is integrated into the underwear and measures the quantity of urine loss on a super absorbent, fast-drying textile (the microfiber consists of three layers).
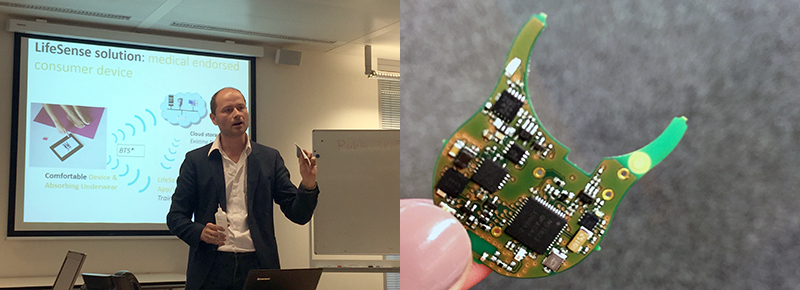
The Carin system is quite an alternative to the current standard of care for incontinence, which includes the use of absorbent pads, surgery, caregiver assistance, and e-coaching. “Incontinence is the biggest taboo in modern society,” said Pop, adding that the company’s goal is for the 400 million people who suffer from incontinence globally to be free of the condition. LifeSense has already had its first sales and started shipping product less than a month ago. The company’s marketing plan is to begin with addressing female urinary incontinence and expand to children between two and 10 years old. The company’s business model is based on the belief that 70% of patients with the condition can be cured, especially women who experience problems following pregnancy.
Holst Centre spin-off Bloom Technologies is developing a medical-grade wearable for pregnancy and prenatal care. Headquartered in San Francisco, the two-year-company has created a technology that provides instant feedback 100 times faster than comparable technology, while being 40 times cheaper and 10 times smaller, according to Torfinn Berset, head of software engineering at the company.

The goal of Belli, the small wearable, is to help women take preventive measures and pave the way to evidence-based clinical algorithms in order to predict and manage pregnancy complications, including pre-term birth (measuring contractions, stress and activity), gestational diabetes (measuring activity, calories and fetal movement) and preeclampsia (measuring stress, activity and fetal movement). Bloom Technologies has partnered with the University of California, San Francisco on the second generation of the app. Belli has been launched in San Francisco and is available to rent at a rate of $29 per week. Berset said the product will be available for purchase by the end of the year, but declined to state its full cost.

Combining ultra-low power electronics with flexible electronics, a health patch developed at the Holst Centre provides a platform for impedance measurement. The fully disposable prototype includes a sensor module that measures just 17.4 x 17.4 mm and a stretchable system to enable better signal integrity without putting force on the electrodes. The real-time electrocardiogram measurement is transmitted via Bluetooth and can be transferred to a smartphone or tablet. Researchers are working on an application for cardiac heart failure patients.