Many device manufacturers struggle with the day-to-day management of product development documentation and traceability, much of which is due to a document-centric and siloed approach. Breaking down silos between departments and ensuring that risks are visible to all team members during the product development process, and in a timely manner, continues to be an issue for companies. And when it comes to managing the development and documentation process with a comprehensive software platform, there can be even more barriers to achieving a streamlined process.
As devices become more complex, it’s important to collaborate across all departments, especially product development and quality, to develop and implement a risk mitigation strategy. “That’s the biggest challenge,” says Larry Nicholson, life sciences business development manager at Seapine Software. “It’s rare that a project involves three or four people. There are usually 30 or 40 people involved, so quite a few relationships are happening at once.” An addition to the actual collaboration between personnel is the integration of an end-to-end software platform that can help streamline product development processes and ensure traceability and visibility throughout the product lifecycle.
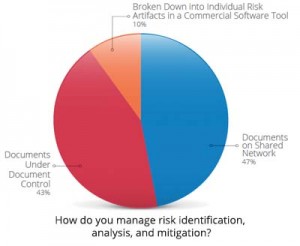
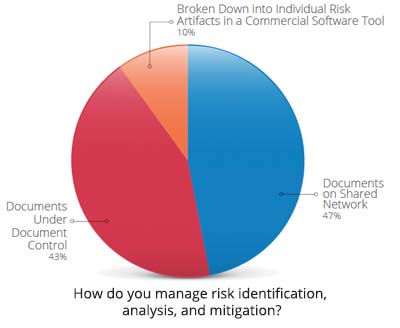
Figure courtesy of Seapine Software
“At best, companies have issue-tracking systems and CAPA systems, but tying them all together and providing the proper visibility with their partners is a challenge,” says Nicholson. “Everyone is using different tools, and that’s half the battle.” For the past six years, Seapine Software has been conducting an annual survey to learn about the top hurdles that device companies face. Last year, the nearly 500 professionals surveyed cited managing product development risk visibility throughout the life cycle, time spent on documentation, and overcoming barriers to improvement as their main challenges.
“If the medical device market keeps going down the path of purchasing software tools to just manage their departmental items or just looking at it from a regulatory standpoint without looking at the bigger picture, there will be issues and delays,” says Nicholson.
For device companies and suppliers that haven’t yet adopted a comprehensive software platform for product development, Nicholson offers three tips to start the process:
- Develop a traceability strategy. A top-down approach is best, but people who can view the project from concept to delivery
- Get all development team members connected. The ability for all team members to see related information or impacted work, especially for complex or high-risk products
- Find balance for all development team members. Get buy-in with process, methods, effective transfers, and tools
This year Seapine Software’s State of Medical Device Development Survey asks questions surrounding connected devices and home healthcare; innovation (or lack thereof); adoption of and confidence in cloud-based solutions, and the impact of emerging technologies. Medical device professionals can participate through August 31.Up and Coming: Becoming Agile
The concern over the implementation of Agile in a highly regulated environment such as the medical device industry prompted AAMI to release a guidance on how to use Agile in developing medical device software. However, that was two years ago, and the adoption of Agile in the medical device industry is growing. In last year’s survey by Seapine, nearly 50% of respondents stated they were either using Agile or hybrid methods, or they planned to adopt them within the year. “Everyone outside the medical device industry is practicing Agile methods,” says Nicholson. “I think the medical device [industry] is in the curiosity stage now. Nobody’s jumping in with both feet, and I’m not sure they need to, especially with high-risk products.”
Some folks in the industry are still learning about whether Agile methods can provide a benefit to software and product development management. AAMI offers a course on using Agile practices in developing device software, covering topics from aligning Agile to the quality system to verification and validation to documentation and traceability.