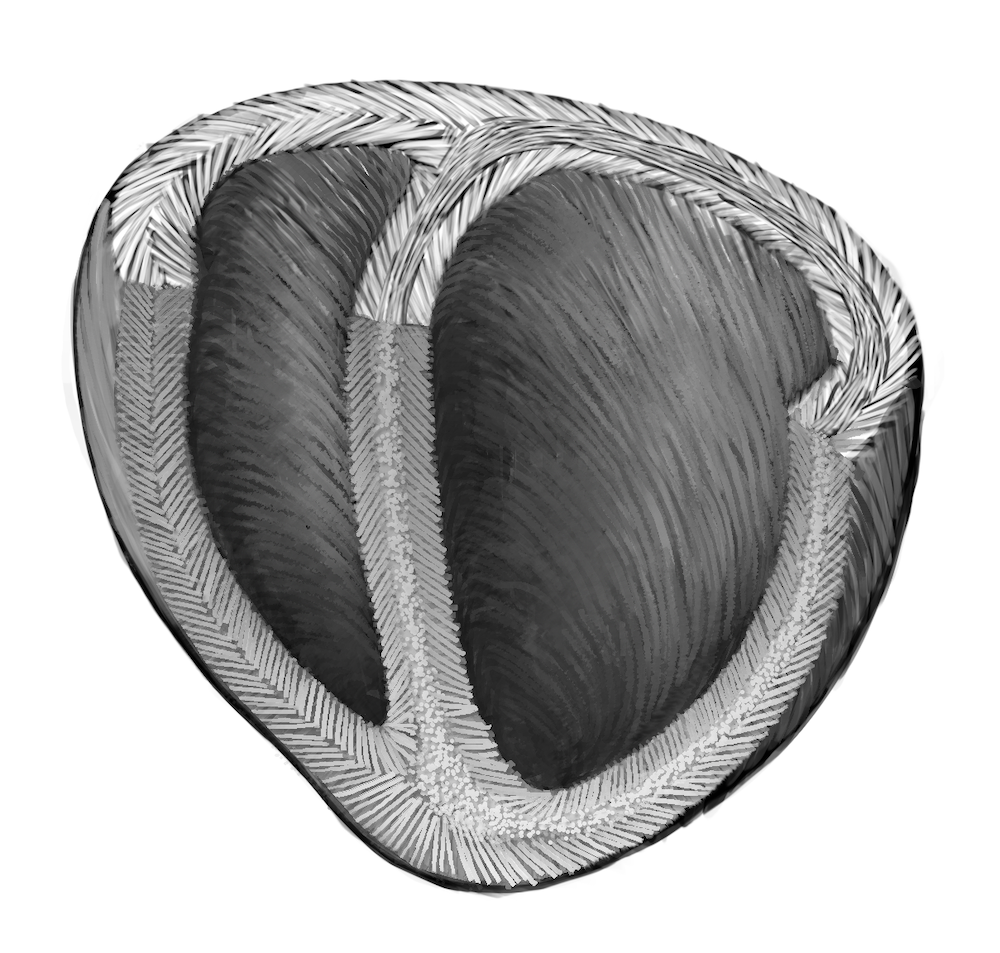
Bioengineers from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) have developed the first biohybrid model of human ventricles with helically aligned beating cardiac cells. Using focused rotary jet spinning (FRJS), which enables the fabrication and deposition of polymeric materials in the form of long, thin fibers ranging from several micrometers to hundreds of nanometers, they were able to fabricate the hearts. They then seeded the scaffolds with cardiomyocytes, enabling the biofabrication of tissue-engineered ventricles.
As reported by Leah Burrows of SEAS, it has long been theorized that the twisting motion of the helical geometries of the ventricles is critical for pumping blood at high volumes. The findings of the SEAS research team, published in Science, showed that muscle alignment does, in fact, dramatically increase how much blood the ventricle can pump with each contraction.
Developed at SEAS by Kit Parker’s Disease Biophysics Group, FRJS fibers direct cell alignment, allowing for the formation of controlled tissue engineered structures. “This work is a major step forward for organ biofabrication and brings us closer to our ultimate goal of building a human heart for transplant,” said Parker, the Tarr Family Professor of Bioengineering and Applied Physics at SEAS and senior author of the paper.
In 1969, Edward Sallin, former chair of the Department of Biomathematics at the University of Alabama Birmingham Medical School, argued that the heart’s helical alignment is critical to achieving large ejection fractions.
“Our goal was to build a model where we could test Sallin’s hypothesis and study the relative importance of the heart’s helical structure,” said John Zimmerman, a postdoctoral fellow at SEAS and co-first author of the paper.
To test Sallin’s theory, the SEAS researchers used the FRJS system to control the alignment of spun fibers on which they could grow cardiac cells.
The first step of FRJS works like a cotton candy machine—a liquid polymer solution is loaded into a reservoir and pushed out through a tiny opening by centrifugal force as the device spins. As the solution leaves the reservoir, the solvent evaporates, and the polymers solidify to form fibers. Then, a focused airstream controls the orientation of the fiber as they are deposited on a collector.
The team found that by angling and rotating the collector, the fibers in the stream would align and twist around the collector as it spun, mimicking the helical structure of heart muscles.
“The human heart actually has multiple layers of helically aligned muscles with different angles of alignment,” said Huibin Chang, a postdoctoral fellow at SEAS and co-first author of the paper. “With FRJS, we can recreate those complex structures in a really precise way, forming single and even four chambered ventricle structures.”
After spinning, the ventricles were seeded with rat cardiomyocyte or human stem cell-derived cardiomyocyte cells. Within about a week, several thin layers of beating tissue covered the scaffold, with the cells following the alignment of the fibers beneath.
The researchers compared the ventricle deformation, speed of electrical signaling and ejection fraction between ventricles made from helical aligned fibers and those made from circumferentially aligned fibers. They found on every front, the helically aligned tissue outperformed the circumferentially aligned tissue.
The study also demonstrated that the process can be scaled up to the size of an actual human heart and even larger, to the size of a Minke whale heart (they didn’t seed the larger models with cells as it would take billions of cardiomyocyte cells).
The study was supported in part by the Harvard Materials Research Science and Engineering Center, the National Institutes of Health with the Center for Nanoscale Systems and the National Center for Advancing Translational Sciences.
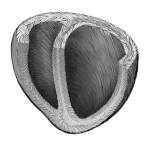
Image: A schematic diagram of the helical alignment of a human heart. (Courtesy of Michael Rosnach/Harvard SEAS)