Smart medical devices refer to a group or products that enhance diagnostics, monitoring and therapeutics delivery for consumers. The digital revolution has taken the healthcare world by storm in recent years, prompting the emergence of technological advancements deemed unattainable in the past. With the world of IT growing at a breakneck speed, innovation in medical technology has surged tremendously, giving rise to a cohesive ecosystem of connected devices used to generate, collect and analyze vast volumes of medical data.
The footprint of this network in the medical domain is vast, with almost 60% of healthcare organizations across the globe having integrated IoT into their operations in some way. This network, also known as IoMT (Internet of Medical Things), is contributing heavily to the rapid transformation of the healthcare sector, with smart medical devices playing a central role.
COVID-19 Testing Requirements Highlight Importance of Smart Diagnostic and Monitoring Devices
The outbreak of the novel coronavirus, or SARS-CoV-2, which quickly snowballed into a global pandemic in early 2020, has challenged the healthcare industry like never before, leaving numerous disruptions and burdens in its wake. Given the high transmissibility of the virus, nations worldwide put into effect stringent social distancing protocols, mandating people to stay home.
In this scenario, digital health and telemedicine witnessed a tremendous surge in interest, as patients began to seek ways to comply with the isolation mandates, without having to compromise on access to healthcare. According to the CDC, the volume of telehealth consultations rose by almost 50% in Q1 2020, as compared to the same period in the previous year.
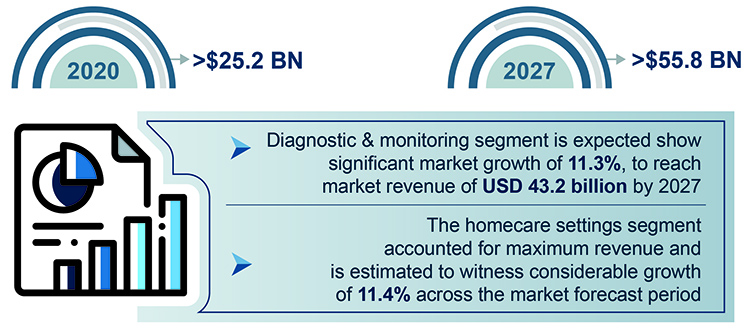
Given the critical role of testing in the treatment of COVID-19 patients, diagnostic and monitoring devices are becoming particularly relevant, with the smart medical devices market from the segment expected to register nearly 11.3% growth rate between 2021–2027, as per projections from Global Market Insights. The respiratory impact of the COVID-19 disease on the lungs is different from that of regular pneumonia and creates oxygen deprivation that is extremely difficult to detect in the early incubation stages.
This factor has drastically increased the importance of pulse oximeters in the pandemic era to help prevent further exacerbation of the disease. In this situation, wearable SpO2 monitoring devices are being used increasingly in hospital as well as home healthcare settings, to monitor patient symptoms and mitigate the risk of conditions like silent hypoxia.
Several smart diagnostic and monitoring solutions were being developed for use in COVID-19 response efforts. For example, in September 2020, Masimo announced FDA authorization for its Rad-G Pulse Oximeter, a rugged handheld device designed to deliver vital parameters like clinically proven SET pulse oximetry and RRp, for continuous monitoring as well as spot-checking. It is equipped with a powerful rechargeable battery, lightweight, strong rubber casing, and convenient direct-connect sensor capable of monitoring SpO2 levels in both children as well as adults. The Rad-G solution was built to simplify patient assessment and care-related decision-making for clinicians.
Favorable Regulatory Framework to Boost Smart Medical Devices Innovation in Europe
The burgeoning presence of smart devices in healthcare is being supported largely by increasing government efforts to enhance patient care and drive innovation in the medical world, with developed economies like Europe leading the charge. According to GMI research, the European smart medical devices market is poised to depict a double digit CAGR through 2027, as a result of the highly supportive regulatory environment for medical technology innovation in the region.
Europe is known for its robust and diversified ecosystem of R&I (research and innovation) in healthcare, with companies and organizations placing a strong emphasis on advancing health diagnostics, smart medical technology and pharmaceuticals, to name a few. These efforts are bolstered by the implementation of initiatives like Horizon Europe, an EU Council, and Parliament-approved incentive program put into effect in January 2021, for R&I in health and healthcare-related sectors.
Likewise, in May 2020, the European Institute of Innovation and Technology introduced the EIT Crisis Response Initiative, as a core part of the European Union’s efforts to tackle the coronavirus pandemic. As part of the initiative, EIT Manufacturing was awarded €3.3 million, to fund promising projects that directly address manufacturing challenges pertaining to smart medical devices.
A notable example of such as project was the INNOV-Ventilator project, led collaboratively by Tecnalia, an EIT Manufacturing Partner, and a consortium of German and Spanish entities including University Hospital of Cologne, TU Graz, WILD, Carl Reiner, Biocruces Bizkaia Health Institute, and Osakidetza. The project was designed to address the issue of ventilator shortage, via the design of a novel, fully pneumatic (air-powered), simple to use, easily manufacturable, and upgradable ventilator device, equipped with advanced display and sensor technologies.
With the advancement of improved sensors and artificial intelligence, a revolution in smart medical devices is on the horizon. It is evident that this revolution will have a huge impact on healthcare dynamics, with the smart medical devices industry poised to expand into something remarkable on the back of persistent advancements in sensor technologies. Smart devices will be relied on by healthcare professionals and patients alike to support telemedicine efforts, as monitoring of vitals becomes easier. Consistent prevalence of obesity and diabetes will sustain the demand for more efficient monitoring devices, augmenting smart technology adoption.