The integration of Real-World Evidence (RWE) in MedTech is reshaping post-market surveillance, offering unprecedented insights into device performance and patient safety in real-world settings. As regulatory bodies increasingly recognize the richness and value of RWE, particularly in informing the benefit-risk profile of devices from real-world environments, MedTech companies are turning to advanced analytical tools to navigate this new landscape efficiently.
MedTech Challenges in RWE-driven Analytics
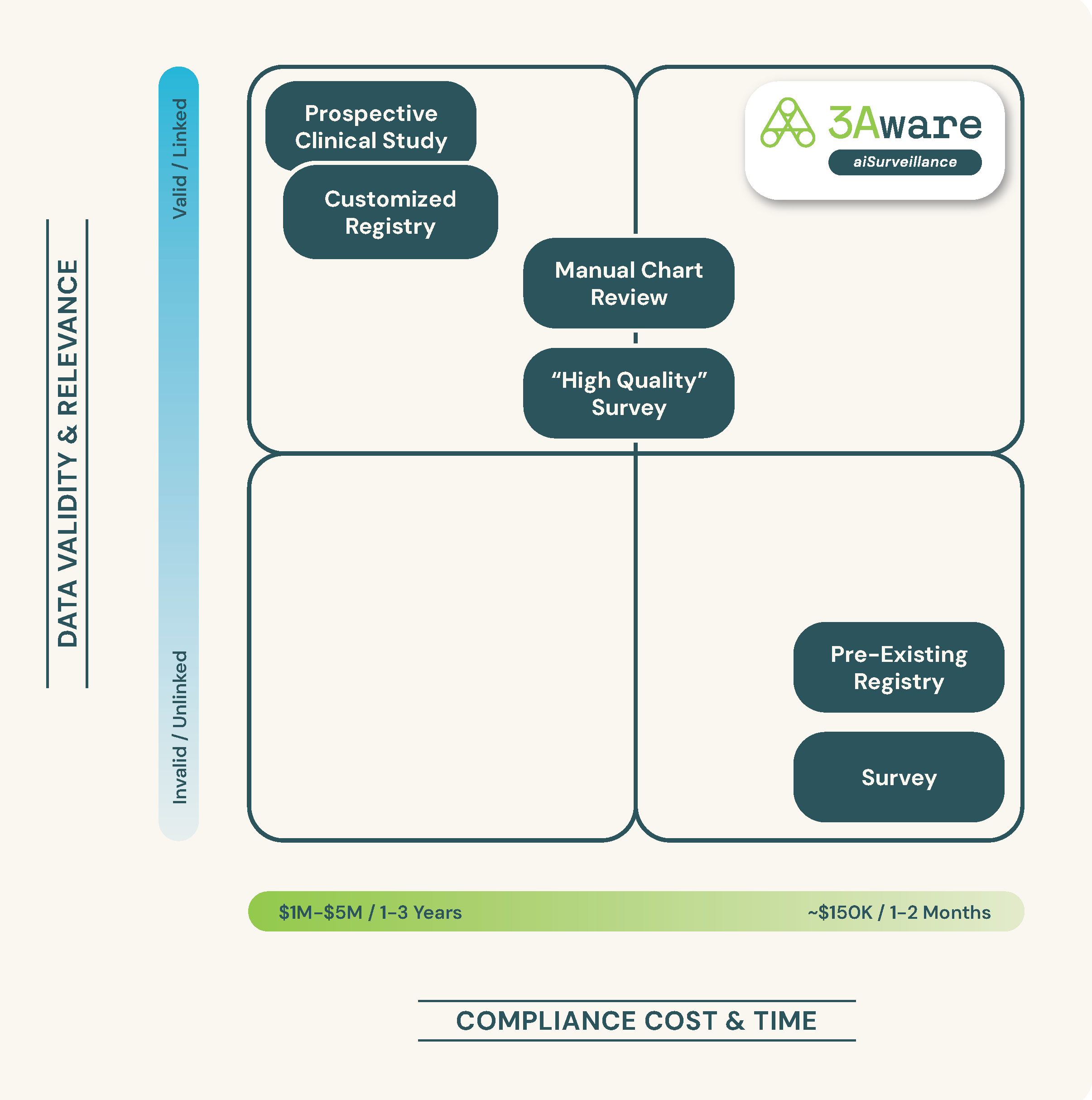
- Complex Data Curation: Managing and synthesizing vast amounts of unstructured and diverse data from various sources, and linking specific device part numbers to patient procedures.
- Significant Time Investment: The slow pace of traditional data analysis methods, which often cannot keep up with clinical demands and regulatory timelines.
- Heavy Reliance on Consultants: Many organizations depend heavily on external experts for data analysis, adding cost and potential variability.
- Data Integration and Quality Limitations: Ensuring data from different sources is consistent and of high quality remains a significant challenge.
Introduction to aiSurveillance
aiSurveillance is a new approach combining data science and clinical science, developed specifically for the MedTech industry. aiSurveillance software integrates real-world data (RWD) sources, such as EHRs and inventory management systems. The software processes vast amounts of patient data, transforming the data into easily assessed patient outcome information from which reliable and actionable insights can be discovered. These RWE insights, available to MedTech at a fraction of the time and cost of other RWE methods, drive timely clinical and strategic product decisions.
How does aiSurveillance Address the Pain Points?
- Automated Data Curation: By automating the curation process, aiSurveillance minimizes human error and manual data handling, and maximizes efficiency, allowing for a more reliable compilation of RWD.
- Enhanced Data Integration and Quality: The platform’s robust data management system ensures that data from various sources is accurately integrated and validated, maintaining the highest quality standards for reliable analysis.
- Reduced Time and Resource Dependency: Its accelerated data analytics capabilities drastically cut down the time from data collection to insight generation. Months and years of work are completed in days and weeks, reducing internal resources as well as reliance on costly consultants.
Potential Impact of Active Surveillance for MedTech
The capabilities of aiSurveillance can revolutionize MedTech by enabling immediate responsiveness to emerging RWD. In the near future, predictive analytics will forecast potential device failures or adverse events, allowing their prevention
before they occur. Ongoing analysis can influence regulatory policies and lead to adaptive safety standards that evolve with emerging RWE. Beyond confirmation of continued safety and performance, new insights can reveal the full spectrum of device utilization, including off-label use.
Technology is set to transform post-market surveillance by enabling MedTech companies to leverage RWE effectively. This shift towards timely, data-driven insights promises not only to enhance product safety and performance, but also catalyze a new era of innovation in medical technology. As the industry continues to evolve, embracing advanced analytical tools like aiSurveillance will be key to driving MedTech forward.
Further Reading
FDA Draft Guidance, “Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices”
Matthias Fink, MD; Tonia Jeiter, MD; Richard Holborow, BSc (Hons); Christoph Ziskoven, MD. Clinical Evidence Under the EU MDR: A Notified Body Perspective. RF Quarterly. December 2023.
Olivia McDermott, Breda Kearney. The value of using real-world evidence as a source of clinical evidence in the European medical device regulations: a mixed methods study, Expert Review of Medical Devices. December 2023. DOI: 10.1080/17434440.2023.2291454
Jay Erturan, MD. Building the Pathway to Successful Use of RWE. BONEZONE. January 2024.
Maria Kamusheva, Bertalan Nemeth, Antal Zemplenyi, Zoltan Kalo, Jamie Elvidge, Maria Dimitrova, Johan Ponten, Konstantin Tachkov, Zornitsa Mitkova. Using real-world evidence in healthcare from Western to Central and Eastern Europe: a review of existing barriers. Journal of Comparative Effectiveness Research. June 2022.







