As medical device manufacturers race to bring innovative new products to market, they must overcome unique challenges—managing product risk, documenting proof of compliance with industry regulations, and streamlining development with new methods and tools. To help manage these challenges, medical device companies have found new ways to improve the product development process. These process improvements—and the challenges that drive them—are what Seapine Software sought to understand with the 2014 State of Medical Device Development Survey.
Managing risk and document management were identified as the first and second challenges by the survey respondents. The third challenge was “Overcoming Barriers to Improvement.”
Despite a clear need to get better development lifecycle management solutions in place, companies are restricted by tight budgets, validation overhead, and bureaucracy. In this Q&A, Matt Harp and Angie Pepiot at Seapine Software provide further insights. 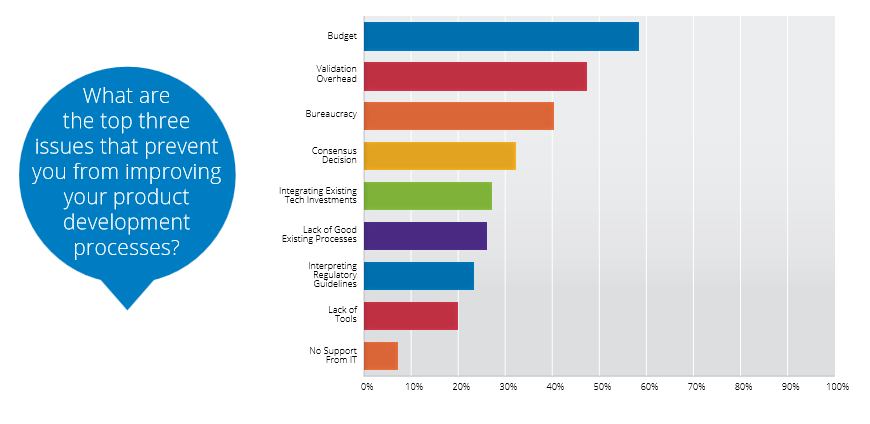
MDS: In your recent State of Medical Device Development survey, “overcoming barriers to improvement” was identified as a key challenge by the medical device industry. What, according to you, are the reasons for this?
There are several reasons why overcoming barriers to improvement is a key challenge for the medical device industry. The top three barriers identified by survey respondents were budget, validation overhead, and bureaucracy. As you can imagine, budget is the biggest barrier to improvement. Developers need new tools to improve quality, productivity, and time to market, but management is often hesitant to spend the money. Unless developers can convince management that new tools will ultimately save money, they’ll remain hobbled by outdated technology.
The cost and time spent validating new and upgraded systems moved into second place—it was ranked fifth in our 2013 survey, when only 27 percent reported validation overhead as a top barrier to preventing improvement. This year, nearly half say it keeps them from improving their product development processes. From talking with our customers, we know that validation is a time-consuming process, or an expensive one—it can take a good chunk of money to bring in an outside expert to do the job. With tight budgets and even tighter deadlines, the time and expense of validating a new system is a big hurdle to overcome. This hurdle often prevents teams from even considering making a change, even though some software manufacturers are working hard to reduce the validation burden for new adopters. Like last year, bureaucracy remained the second-biggest barrier to improvement for managers, but it was also high on the list for non-management respondents. Many companies have complicated rules and processes that stand in the way of improving their development processes. This makes some sense when it’s tied to ensuring quality and regulatory approval, but we hear from a lot of companies where it feels like rules and regulations get stuck in place even when the underlying reasons for having them no longer apply.
MDS: What are some thoughts on how companies can better balance innovation and regulatory compliance?
From our research, it’s clear that proving compliance often eats up a lot of time that developers could be using to improve the product. Nearly half of survey respondents reported wasting a day or more each time they need to update the traceability matrix. There are newer tools and technologies that can automate compliance activities and traceability, doing in minutes what takes teams days with older methods and tools. Companies should also regularly review policies to ensure they still make sense, and investigate ways to reduce the burden of those rules without reducing the intent.
MDS: What do you think about Validation being identified as a key barrier to improving the product development process?
It’s something our customers have struggled with for years, so it’s not too surprising. Validation is a legitimate issue when an organization has to spend money on a contractor to help or a team has to take time out of a real business project to document the system in use. If you think about it, it’s really kind of “busy work” in the sense that the team is just documenting what they’ve already done. On the other hand, some tool vendors are making changes and adding features to address this challenge. We hate to hear about situations where an organization won’t even consider a change because of their fear of validating the new system— it’s often a hill to climb, and not a mountain. So you need to be aware of the real challenge without letting it cause paralysis, where outdated systems and practices remain in place when there are better alternatives available.
MDS: What are some steps that medical device companies can take to overcome these barriers to improving their product development process?
To deliver innovative products within reasonable timeframes, you need to invest in the tools that give your product teams the time and energy to be innovative. Look at the points in your development process that take the most time to complete. In the medical device industry, those are most likely to be the compliance activities—maintaining traceability, linking requirements to source code and testing to requirements, and so forth.
Once you identify these time sinks, find ways to streamline or eliminate them. You might need a new development process (e.g., Agile or some hybrid of Agile and your current process), or just a new tool. If the compliance effort relies heavily on shared documents, you may need to adopt a more artifact-centric process. If it takes several hours or days to create and maintain a traceability matrix, you may need a tool to automate that process.
Once you know where your process bogs down and what you need to do or purchase to fix it, then you can determine if the return on investment is worth it. Then, you’ll be in a stronger position to make a solid case for including new technology in the budget and overcoming the resistance to implementing it.





