Why Are the New FDA Regulations Important for LDTs?
Historically, laboratory Quality Management System (QMS) requirements have been governed by CLIA (Clinical Laboratory Improvement Amendments) and CAP (College of American Pathologists). The new FDA regulations introduce stricter compliance measures, requiring laboratories to meet the FDA Medical Device QMS requirements outlined in 21 CFR 820.
Key Changes Under the New FDA LDT Regulations
The FDA’s approach to regulating LDTs is phased, with different compliance requirements set for the next three years:
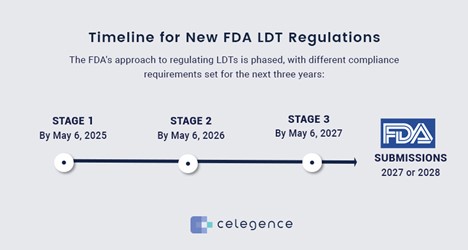
- Stage 1: By May 6, 2025 – Laboratories must update their QMS to include adverse event reporting, reporting of corrections and removals, and establishing a complaint file.
- Stage 2: By May 6, 2026 – Compliance extends to completing a registration and device listing, updating regulatory labeling, and adhering to investigational use requirements.
- Stage 3: By May 6, 2027 – Full QMS updates are required, incorporating design controls, purchasing controls, acceptance activities, CAPA (Corrective and Preventive Actions), and comprehensive record-keeping.
- FDA Submissions: 2027 or 2028 – Depending on the LDT’s risk classification, submissions to the FDA must be made by November 6, 2027, for PMA (Premarket Approval) pathway tests or by November 6, 2028, for tests following the 510(k) or De Novo pathways.
Immediate Steps for LDT Compliance
Adverse Event Reporting
Labs need to develop robust systems to report any adverse events to the FDA. For instance, if a specimen collection device causes a patient rash, this incident must be reported.
Corrections and Removals
Procedures must be established for reporting corrections and removals. If a lab test result is voided due to a lab error, it falls under this category and must be documented accordingly.
Complaint File Management
Laboratories must maintain a complaint file, tracking and resolving complaints made directly or found on social media and other platforms. For example, an Instagram post from a patient claiming a lab test error must be addressed per FDA regulations.
LDT Legal Challenges and Industry Concerns
The American Clinical Laboratories Association (ACLA) has raised concerns, filing a lawsuit against the FDA, arguing that the new regulations overstep the FDA’s authority. While this legal battle unfolds, laboratories must prepare for compliance, as significant changes will require substantial investment, particularly for FDA submissions scheduled in 2027 or 2028.
Strategic Preparation for LDT Compliance
Start with a Gap Assessment
Conducting a gap analysis to assess your current QMS against FDA requirements is recommended. Early identification of compliance gaps allows for a smoother transition to meeting new standards.
Phased Implementation
Gradually implement new procedures to bridge gaps in compliance. This approach minimizes disruption and helps laboratories adjust to new requirements over time.
Staying Ahead: Why Early LDT Compliance Matters
While some laboratories might consider delaying compliance efforts due to ongoing legal challenges, early preparation offers several advantages:
- Cost Efficiency: Bridging QMS gaps early involves relatively low investment compared to the potential costs of last-minute changes.
- Risk Mitigation: Proactive compliance reduces the risk of penalties, product recalls, and market disruptions.
- Regulatory Assurance: By aligning with FDA requirements, laboratories can confidently offer LDTs that meet the highest standards of safety and quality.
MedTech Intelligence Content Partner
![]()
This blog post was previously posted by Celegence LLC. The author is employed by or otherwise directly associated with this Partner.







