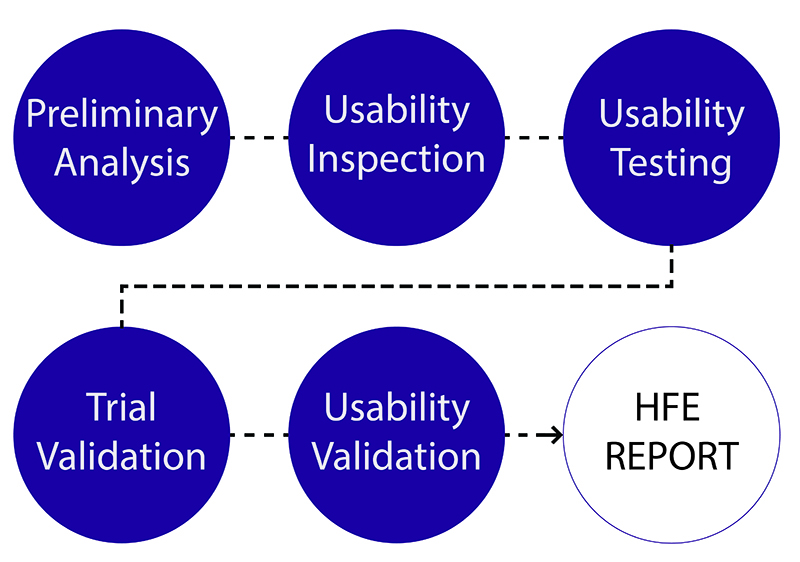
A missed opportunity: That’s what we call it when an otherwise great device is saddled by a mediocre user experience. In view of strong competition and equivalent functionality, a new medical device’s success may very well ride on its ability to deliver a great user experience (UX). Oddly, however, few medical device manufacturers invest heavily in user experience design. Rather, they take a technology-centric development approach that might or might not lead to a user-pleasing solution. Or, they try to add user-friendliness too late in the design process and in superficial ways that stand little chance of matching the user interface quality that could have been achieved by a more comprehensive effort.
Indeed, when human factors engineering (HFE) is not integrated fully into the product development process from the start, manufacturers are likely to discover during late-stage development activities that users struggle to complete essential and critical tasks in an error-free manner. Ultimately, critical use errors become a roadblock to gaining regulatory approval for the given device. Specifically, a manufacturer might not be able to generate evidence during a summative (i.e., validation) usability test of safe and effective device use—usually a prerequisite for approval.
In many cases, summative usability testing reveals use errors that might be avoided by making a significant user interface design change, but the design has already been “frozen.” This posture has led many manufacturers to regard enhancements to “information for safety” —labeling and/or training—as an alternative way to prevent significant use errors. Unfortunately, such enhancements are rarely an effective fix when there are hardware and/or software design shortcomings at the root of such use errors. Moreover, FDA and other international regulators explicitly state that “information for safety” is the least preferred and least effective risk mitigation strategy, and EN 14971:2012 (a European harmonized version of ISO 14971, the risk management standard) states that “manufacturers shall not attribute any additional risk reduction to the information given to users.”
Consequently, manufacturers often must “thaw” their frozen designs and fix them to eliminate the root cause of significant use errors. Of course, “thawing” a user interface design is expensive and time consuming, which explains manufacturers’ reluctance to do so.
The key to avoiding such pitfalls and developing a great user experience is to incorporate HFE into product development from the start, including during design conceptualization. By applying fundamental HFE and UX design principles, HFE specialists, both in-house and third parties, can help designers avoid common mistakes, such as not grouping related controls, using unfamiliar terminology, or not implementing confirmation screens that seek users’ confirmation of critical actions. Importantly, an HFE specialist’s contributions should extend past a device’s hardware and software user interfaces to every device touch point, including packaging to labeling. Take the example of a dialysis machine where a user must install and connect multiple disposable components in a particular order. An HFE specialist might suggest the following:
- Ensure there is a handle that enables users to carry the disposable kit over to the device using one hand.
- Ensure there is an affordance (e.g., a conspicuous perforated pull tab) that makes the outer packaging easy to open and unwrap.
- Package the disposable components such that the component that needs to be installed first is located on top, increasing the likelihood that users remove that item first.
- Ensure that each component’s expiration date is presented conspicuously and in an unambiguous format (e.g., “12 Dec 2017” instead of “12-11-17”).
- Number each component and the associated connection point on the device, enabling users to quickly determine where to install each component and in which order.
- Color- and shape-code connections to prevent misconnections.
- Integrate sensors into the system and provide tactile, audible, and/or visual feedback indicating whether a component has been installed correctly.
- Develop a software user interface that uses clear animations and illustrations to guide users through the installation process, reinforcing the aforementioned numbering and color-coding schemes.
In a previous article, we discussed various models for engaging HFE resources. Whether they work “in-house” for a medical device manufacturer or as external consultants, HFE specialists and UX designers can add substantial value at every stage during device development. In addition to formative and summative usability testing, arguably “obvious” times to engage HFE specialists, some of the key activities that HFE specialists can perform throughout the product development process include the following:
- Develop HFE plans. Develop a plan at the very start of each development effort that outlines how HFE will be applied throughout device development. Having an HFE plan helps ensure that all project stakeholders are “on board” with performing HFE, ensures that the right set of HFE activities will be performed, and increases the likelihood that the necessary time and money is allocated to such activities.
- Benchmark similar devices. Critique predecessor and competitor devices to identify each device’s strengths and shortcomings. These findings enable manufacturers to “cherry pick” the best aspects of existing devices, identify opportunities to improve upon the existing user experience, and gain a competitive advantage in the market.
- Contribute to task and use-related risk analyses. Conduct a “PCA” task analysis to understand where a break down in users’ perceptions, cognitive processes, and actions might lead to use errors, and help conceptualize design-based mitigations to prevent such use errors. HFE specialists can also help analyze workflows to understand where automation or combining steps can reduce the burden on the user, thereby creating safer and more efficient interactions.
- Participate in design reviews. Support user interface (UI) and industrial designers by reviewing an interface’s evolving design in the context of HFE best practices. Even in the concept development stage, HFE specialists can critique sketches, wireframes, and/or draft user documentation to identify opportunities for improvement and discuss usability and use-safety tradeoffs of competing designs. Such feedback is particularly valuable early in device development, when HFE specialists can identify and help resolve “low-lying” user interface shortcomings, thereby enabling designers to focus on more difficult design challenges and decisions.
- Contribute to UI requirements. Conduct user research (e.g., observations, interviews) to identify unmet user needs and collaborate with engineers to translate user needs into verifiable and realistic requirements that leave room for creativity and innovation while ensuring the final design meets users’ needs.
- Develop training materials and labeling. Help conceptualize different content delivery strategies, identify information needs and learning goals, and evaluate the design of training materials and labeling (e.g., quick reference guides, instructions for use) to ensure they are clear, concise, and appropriately integrated into the overall user experience. Training and labeling materials are often treated as afterthoughts despite the fact that such materials can be paramount to a user interacting with a device safely and effectively.
HFE specialists are uniquely qualified to help manufacturers design a user experience that ensures safe and effective use and gives a device a competitive advantage. Applying HFE from the very start of a device development effort—and performing the right HFE activities at the right time—is essential to ensure design decisions are driven by users’ needs. Ultimately, a device that meets users’ needs will be a device that meets the manufacturer’s needs by leading to increased sales, increased customer satisfaction, and reduced risk of adverse events.