CDRH Director Jeff Shuren, M.D. shared the secret to removing your company from FDA’s plan for routine inspections at the latest Case for Quality (CfQ) forum.
The answer? Join the Case for Quality movement and demonstrate that your company provides patients access to high quality, safe and effective medical devices. To succeed, your company must share the FDA goal to provide patient access to high-quality medical devices. The CfQ initiative has now matured into a CDRH Strategic Priority.
How Did We Get Here?
“We have heard that too much of a focus on compliance with FDA regulations, rather than on device quality, drives some companies to focus on making FDA inspectors happy and checking the quality system regulation requirements boxes rather than focusing on innovating around device quality,” commented Shuren. “The Agency realizes that in the past, the focus of the relationship between FDA and industry may have been on managing compliance rather than on a shared goal for continuously improving quality.”

Shuren describes this approach as Whack a Mole.
“In part, in response to this perspective, for which we believe there is some truth, as well as that the use of our traditional enforcement tools have not moved the needle in terms of advancing device quality, we launched our Case for Quality initiative,” he added.
What Is the CfQ Vision?
Achieving the vision of CfQ requires a paradigm shift for all stakeholders in the realization that compliance with device regulations does not ensure high product quality.
To improve product quality, we must develop, define and implement quality metrics across the ecosystem. The CfQ is about creating a competitive marketplace for device quality and providing market and regulatory incentives for device manufacturers to assure they produce high-quality devices consistently and reliably.
What is the Value of Quality and Why Should it Matter to You?
Quality results in improved patient outcomes. From a business perspective, competing on quality can result in higher revenues, resulting in opportunities for increased innovation investment.
The agency realizes that corporate culture plays a key role in quality. While FDA does not regulate culture, it does recognize its impact.
FDA would reward top performing companies that adopt CfQ practices for high quality as follows:
- Remove top performers from the agency’s work plan for routine inspections (no more FDA inspections)
- Work to identify non-regulatory solutions when issues occur
- Forego pre-approval inspections
- Not require 510k’s for certain changes
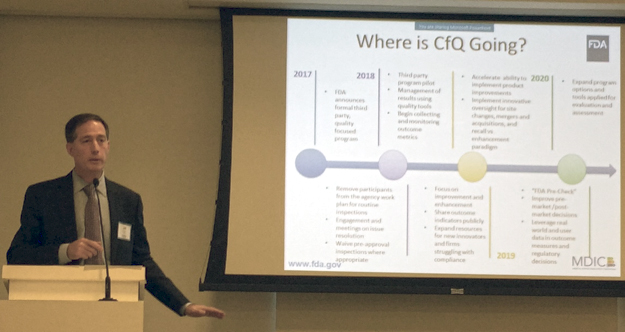
Shuren detailed the following CDRH action plan in support of the CfQ Initiative:
2017
- FDA announces formal third party, quality focused program
- Remove participants from the agency work plan for routine inspections
- Engagement and meetings on issue resolution
- Waive pre-approval inspections where appropriate
2018
- Third-party program pilot
- Management of results using quality tools
- Begin collecting and monitoring outcome metrics
- Focus on improvement and enhancement
- Share outcome indicators publicly
- Expand resources for new innovators and firms struggling with compliance
2019
- Accelerate ability to implement product improvements
- Implement innovative oversight for site changes, mergers and acquisitions, and recall vs. enhancement paradigm
- “FDA Pre-Check”
- Improve pre-market /post-market decisions
- Leverage real world and user data in outcome measures and regulatory decisions
2020
- Expand program options and tools applied for evaluation and assessment
What’s the Secret to Stopping FDA Inspections?
The secret to stopping FDA inspections and foregoing pre-approval inspections can be distilled into one word: QUALITY. After all, you cannot have safe and effective product without consistent quality.
FDA and industry have developed a framework that creates a competitive marketplace for device quality and provides market and regulatory incentives for device manufacturers to ensure they produce high-quality devices consistently and reliably.
By consistently showing a commitment to high quality, instead of simply meeting regulatory requirements, medical device organizations can demonstrate that they provide patients access to high quality, safe, effective medical devices.
In turn, FDA would reward top-performing companies that adopt CfQ practices for high quality by removing them from the agency’s work plan for routine and pre-approval inspections among other regulatory incentives.
The formula to stop FDA inspections is simple: Demonstrate your commitment to providing high quality medical devices.