Shuvo Roy, Ph.D., Professor of Bioengineering at UCSF and Technical Director of The Kidney Project, and his research partners have developed an artificial kidney constructed of semiconductor silicon wafers that remove waste and toxins from the blood and a cell therapy unit that replicates other kidney functions. Their prototype, powered entirely by blood pressure, filtered blood and created urine in a pre-clinical trial. We spoke with Dr. Roy about the device and The Kidney Project’s next steps following its proof-of-concept trial.
Successful development and commercialization of an artificial kidney would be life-changing for so many people. What is the current impact of end stage kidney disease in the U.S.?
Dr. Roy: In the U.S. we have about 800,000 patients who have kidney failure. Worldwide that number is estimated at 3 to 4 million. If you look at chronic kidney disease—patients who are at risk of kidney failure—that’s close to a billion people worldwide and almost 40 million Americans. It is one of the top 10 killers in the world.
If you are unfortunate enough to get to stage 5 kidney failure, there really are only two viable options: dialysis or transplant, which is the better option. But there is a scarcity issue with transplants. We have 100,000 patients on the wait list and only about 25,000 transplants are performed a year. There are also about 500,000 patients on dialysis right now.
This device is unique because you’re not trying to grow a new kidney. Can you tell us a little bit about what this device is and how it works?
Dr. Roy: There are two main approaches right now: one is to grow a new human kidney and the other is to use pig kidneys. We have taken a fundamentally technology-first approach. It is a hybrid approach where you combine a mechanical filter unit with a cell therapy unit, and these two work in tandem to provide the key functions of a healthy kidney.
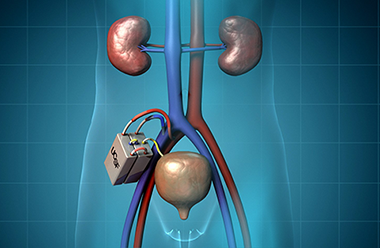
For the mechanical filtration portion of the unit, we are using semiconductor silicon technology to make a highly efficient membrane that runs off blood pressure alone and generates filtrate that has toxins, salts and solids and lots of water. That filtrate gets directed to the cell therapy unit, which has a line of kidney cells that process the filtrate and the water and pump some of it back into the bloodstream. The toxins are concentrated in a small amount of fluid that become urine. This is a totally implanted device that is connected to blood vessels very much like in a transplant and then to the bladder to direct the urine out.
Are you using autologous cells?
Dr. Roy: We’ve been using human cadaver cells. Our silicone membrane technology allows oxygen and nutrients and wastes from the cells to be exchanged, but the pores of the membrane are so small that antibody cells cannot get in and attack the encapsulated cells. So there is no need for immune suppressant or anti-rejection drugs, and this is important because immuno-compromised patients are especially vulnerable. By not needing anti-rejection drugs you avoid that challenge; you also avoid another challenge which is the financial cost of taking immunosuppressant drugs after your transplant.
Where did this concept come from?
Dr. Roy: I am a semiconductor engineer. I am not a physician or a biologist. My training was in electronics and sensors, and I decided to go into bioengineering and look at ways to apply these technologies to medical needs. I met a budding nephrologist, Dr. William Fissell, and he said if you are looking for something to work on, you could look at making life better for dialysis patients. He took me to the clinic and showed me a dialysis machine, which is the size of a refrigerator, and I thought yes I could make this smaller with miniaturization.
As we kept having conversations, we both realized that we could make better dialysis machines as a Band-Aid, but these patients need more than dialysis. Dialysis provides 10% of kidney function. Even then, it doesn’t address the other functions of a healthy kidney, so these patients are always teetering between life and death. The survival rates are poor, and compliance after a few years is poor.
The idea was, we can make a new dialysis machine but how else can we advance the health of these patients? We focused on work that had just been reported by the University of Michigan, where a nephrologist, Dr. David Humes, was treating kidney patients in the ICU by combining a dialysis filter with cell therapy, and the results were incredibly encouraging. He doubled the survival rate of these patients.
Dr. Fissell and I realized that this hybrid approach of mechanical filtration with tubular function by renal cells, which is the architecture of the kidney, was likely the fastest way to get this type of a treatment to patients. That is what inspired our work.
We looked at what Dr. Humes was doing and said if we can make the filter smaller and more compact using a fundamentally different filter technology that also protects the cells, then we can make something small and compact enough that it could be located via surgery very much like kidney transplant within the patient.
Where are you now in terms of the development and testing of the device?
Dr. Roy: We are implanting the device in pigs to show that we can filter blood and generate urine. We have been able to make functional prototypes that produce urine in pigs without the need for anti-rejection drugs. We’ve optimized the chemistry of the surfaces and the blood flow paths, so they do not need anticoagulation therapies, and we’ve been able to show that the pigs are not displaying adverse effects, and the device does not adversely react to the pig either.

The functional prototypes are working, but they don’t have enough capacity to treat the pig. Going forward, we need to show that the device can provide enough therapeutic capacity to keep a human or sick pig alive. We have to increase the surface area of the filter and the number of kidney cells inside the device, and do it in a way that we can keep the same footprint of the device.
So you’re looking at ways to increase the capacity of the device based on your findings so far?
Dr. Roy: That is correct. It’s about design refinement and scale up. We’ve shown that we can filter and generate urine, and the urine comes out in the catheter to the bladder. These are little driplets of urine, but nonetheless clear fluid after processing the blood. That part is exciting, we just need to have more volume.
Healthy kidneys filter about 144 liters of blood into filtrate, and the tubular components of the kidney reabsorb much of that water—almost 143 liters, so that you do not become dehydrated. The toxins are all concentrated in the one to two liters of urine that humans produce a day. We don’t have that volume of urine, but our filtration proportion is in the high 90s, and that is what makes us excited to say okay this is working. We have more rigorous experiments that we have to do, but this is the first evidence.
Could you tell me a little bit about Kidney X and how they are supporting your device development?
Dr. Roy: Kidney X is a prize competition, and we just received our fourth prize. It is a public-private partnership between the Department of Health and Human Services and the American Society of Nephrology. It was created some years back to shine a light on the need for innovations in kidney disease and specifically to catalyze the development of technologies for kidney failure, including the development of an artificial kidney.
We have a disease that affects about 800,000 Americans, and the mortality rate for patients on dialysis is much higher than most cancers. It’s also very expensive. Our government spends close to $50 billion just on end stage renal disease. So, we’re talking about not only improving patient health but also large savings.
The dialysis field hasn’t advanced for the past 50-plus years, and the people who suffer the most are the patients. It will require a public-private partnership to advance technology to that point of becoming inevitable.
What’s so exciting to us is also the equity issue. I make an analogy to the cell phone. I come from a country in East Africa where we largely did not have phone lines. But today we have cell phone service and that has democratized access to information. An artificial kidney has the potential to do that—anyone who needs treatment can get one.
Getting an artificial kidney will still cost money, so do cell phones. But when you look at the economics of scale, we have 70 years of infrastructure investment into reliable and cost-effective semiconductor manufacturing, so the same paradigm as cell phones might apply to this device.







