Many medical devices are subjected to thorough characterization during the design stage to ensure device quality. However, in manufacturing, assembly process variation, supply chain component deviation, test system repeatability and operator handling errors can introduce failure into the device. Some of these defects may not be detected during manufacturing test due to lack of coverage in the test system. Marginally passed units may cause field failures during actual usage due to degraded performance.
To stay competitive in the market, manufacturing tests are normally optimized to achieve low cost-of-test with fast test time to meet market cost expectation. The device may only be tested under certain minimum conditions deemed sufficient. For instance, a wireless medical device OEM recently faced issues with the effectiveness of their manufacturing test setup. A custom version of the Bluetooth Low Energy (BLE) device managed to pass all manufacturing tests but was later found to have intermittent connection issues. After troubleshooting, it was found that device had a distorted antenna pattern, causing much lower power in some of its BLE channels. In production testing, only a very simple connection test was performed that was not able to catch these intermittent connection issues during actual operation.
Capital Equipment Cost vs. Potential Saving
A defect that is detected during initial manufacturing phases may not cost a lot to fix. However, the cost increases exponentially as the detection happens after production test or in the field during end user’s applications.
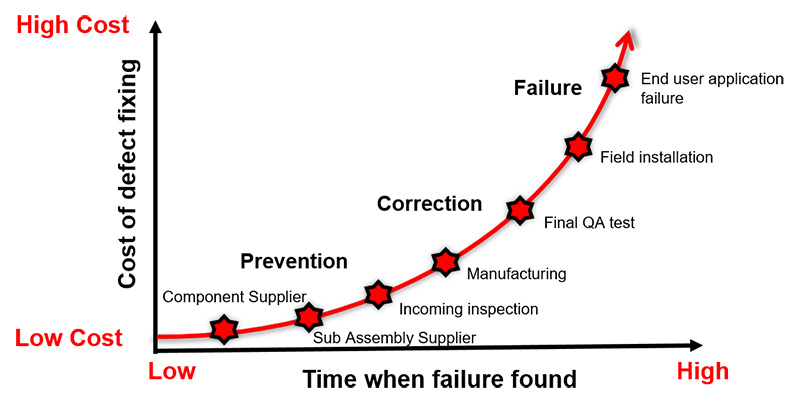
Investing in the right test solution is important during the new product introduction (NPI) phase. It may look expensive to invest in an RF tester and the required operators to execute the tests correctly. There are also annual maintenance and calibration costs. However, the potential savings from detecting the failure early during manufacturing saves the direct and indirect or hidden costs of a field failure. The hidden cost due to warranties, failure troubleshooting, handling of replacement units, loss of sales due to bad reputation, or even penalties arising from use of defective products, can potentially total huge amounts of money.
With the correct test strategies implemented in production, manufacturers can easily recoup the initial RF tester investment within the first year. The table below shows an example of potential cost savings. With the right test strategy, the potential cost saving can go up to $250,000, with savings in warranty cost, and indirect/hidden cost in the event of shipment recall and so forth.
| Example of Potential Cost Savings Analysis | ||
| Example DUT | Patient monitor | |
| Annual volume | 100,000 | |
| Yield rate | 99% | |
| Bad DUTs | 1000 | |
| Warranty cost per unit | $150 | |
| Total warranty cost | $150,000 | |
| Indirect or hidden cost | $100,000 | Costs associated with shipment recall, RMA process, support, shipping, receiving, tracking, corrective action, change orders, legal, and reputation loss. The cost would be far more than $100 per failed unit. |
| Potential cost savings | $250,000 | |
In the next section, we will discuss how leading medical device manufacturers are optimizing their manufacturing tests to ensure device quality, increase manufacturing yield, improve test system flexibility or improve manufacturing throughput.
Case Study 1: Ensure Quality of BLE Wireless Charger
It was the first attempt for a medical device company to incorporate wireless connectivity into their products. This company was developing a BLE enabled wireless charger. By enabling wireless connectivity to the charger, users can easily monitor the charging state and the battery level in order to prolong the battery life. During the design phase, the engineer needed to validate that the antenna and the matching circuits were performing according to the design goals. As the product was developed using an RF module, the engineer skipped the full parametric testing according to the Bluetooth requirements. The RF performance was guaranteed by the module maker. As the engineer made modifications on the reference design and the antenna to fit into their form factor requirements, the engineer had to run full validation at the end device level to ensure the device was transmitting and receiving BLE signals properly in various end user scenarios. The company used an over-the-air (OTA) wireless tester specifically designed for IoT applications, to perform transmitter output power measurements and receiver packet error rate (PER) and sensitivity measurements. The engineer used OTA measurements to validate the overall device transmitter and receiver performance including the antenna. The engineer could also choose to test all 40 BLE frequency channels, or selectively test any channels of interest. With this capability, the engineer could validate the performance of the radio covering the entire BLE frequency band.
The manufacturer also uses the same test setup in manufacturing test, as it is cost-effective and simple enough for operator use. The production test is optimized by performing TX power and RX PER test at only three frequency channels—the lowest, middle, and highest frequency channels, to quickly validate the device performance over the entire BLE frequency band. This has helped the medical device manufacturer accelerate production and minimize correlation issues that frequently happened due to different test setups being used in design and manufacturing.
In this case, the manufacturer has saved weeks of test development during the pilot phase, reduced time-to-market, and ensured device quality by adopting an effective test solution that offers the required test coverage.
Case Study 2: Improve Yield for Wirelessly Controlled Surgical Machine
A manufacturer faced yield issues with their high-end surgical machine, which includes a wireless subsystem for remote control purposes. The wireless subsystem worked properly until failures started to show up. This became a big problem and impacted their shipments, as they only discovered failures after the complete machine was built and tested. When the subsystem failed, they had to spend a long-time troubleshooting, repairing, and retesting. This caused inventory pileups and shipping errors. To fix this problem, they used a simple and cost effective IoT signaling tester to do a pre-screening test of the wireless modules before they were installed into the wireless subsystem in their machine. Identifying defect modules before they were installed gave the manufacturer tremendous savings in test and repair time. This eventually allowed them to meet their daily output and yield targets.
Case Study 3: Improve Production Flexibility for Contract Manufacturer of Low Volume Medical Wearables
A leading contract manufacturer manufactured medical wearables for many different brands. Their existing test solution was based on a non-signaling one-box-tester. Because the test was conducted in non-signaling mode, special test firmware had to be loaded to the device before testing. Then, it had to be removed and replaced with final production firmware after the test was complete. The maintenance of these large sets of firmware for different products were painful for the manufacturing team. Operator handling error was also a key risk for them as they manufactured a wide range of devices from different customers. To improve the production flexibility and eliminate operator handling errors, they switched to an IoT wireless tester that enabled signaling OTA testing using final production firmware. This helped them to streamline the test processes and allowed them to easily switch between different product versions or brands. The tester also supported major short-range formats like BLE 4.2, BLE 5, WLAN 2.4-GHz and 5-GHz so they could use the same test setup to test devices with different radio formats. Production flexibility is important for contract manufacturers to cater to volume fluctuations from their customers.
Case Study 4: Increase Manufacturing Throughput and Reduced Cost-of-Test of High-Volume Wearables
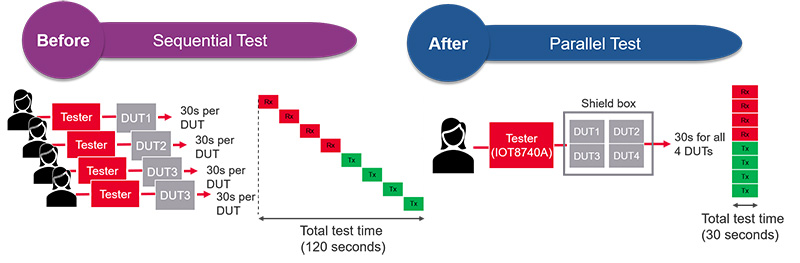
A leading wearables manufacturer was investigating their next generation test platform. Part of their goal was to achieve higher throughput and reduce cost-of-test without sacrificing test coverage. They understood that quality is cannot be compromised in the medical device industry. Their existing test solution was time consuming and operator intensive. It involved manually putting the DUT into the shield box, running the test, removing the DUT from the shield box once the tests completed, inserting a new unit, and repeating the process. It was running in sequential mode. By switching to a test solution that enabled parallel testing of multiple devices, test time was significantly reduced. The operator can put four devices into the same shield box at one time and run the required TX and RX tests simultaneously on all four devices. Once the tests are completed, the operator removed all of them and replaced them with four new units to continue the test. With parallel testing capability, the manufacturer managed to cut test time by more than four times, resulting in significant throughput increases and cost of test reductions.
Conclusion
Connected medical devices are on an exponential growth trajectory. Adding wireless connectivity to medical devices brings tremendous convenience to patients, allowing better healthcare delivery and lowering healthcare costs. The success of this megatrend will depend on the ability of medical device manufacturers to produce reliable, quality-connected medical devices that will not fail prematurely in the field. Effective manufacturing tests play a critical role in ensure the device quality by capturing defective or marginally passed devices that can potentially fail in the end user application. Selecting an effective test strategy will help to minimize this risk without incurring high manufacturing cost.







