

Jordi labs and its team of Ph.D. analytical chemists developed a proprietary, multi-detector approach to ensure that all extractables are accurately characterized to comply with global materials testing regulatory requirements.

Jordi labs and its team of Ph.D. analytical chemists developed a proprietary, multi-detector approach to ensure that all extractables are accurately characterized to comply with global materials testing regulatory requirements.

Taking these compliance challenges into account will allow healthcare organizations to prepare for compliance audits, but more importantly it will help institutions address issues that result in a negative patient outcome.

Peter O’Blenis, CEO of Evidence Partners, discusses the growing role—and challenges –of literature reviews in the medtech market.

Elcam Medical is the first company in Israel to meet the audit requirements set by MedAccred subscribing members.

Steps to ensure your medical device labels are in compliance with global regulations and have the longevity to withstand your device’s intended use.

Gain insight into the notified body approach to assessing biological equivalence and how manufacturers can make sure their equivalence justifications pass notified body review.

The final rule from the FDA establishes a new category of over-the-counter hearing aids, enabling consumers with perceived mild to moderate hearing impairment to purchase hearing aids directly from stores or online retailers.
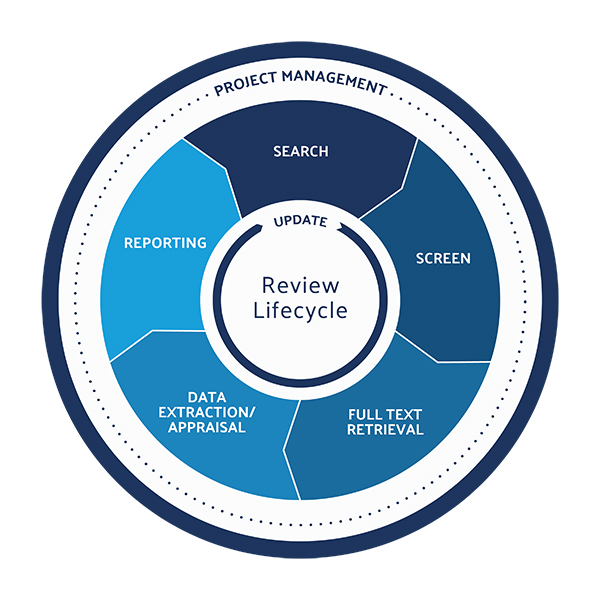
Double-digit growth of the company’s literature review software platform, DistillerSR, spurred $20 million in funding from the growth investment consortium led by Thomvest Ventures.

Companies can make mistakes during drug and device development and in the post-marketing maintenance phase, but all too often, organizations apply a temporary solution to the problem and move on. In this article, Helen Lowe at Arriello addresses how Corrective and Preventive Action (CAPA) management provides a better approach to discover the root cause of the problem.

The Experiential Learning Program (ELP) provides CDRH review staff with an opportunity to visit sites and gain a better understanding of the products they review. The 2023 ELP Site Visit Proposal period is open now until September 6, 2022.