

The acquisition expands MilliporeSigma’s portfolio to a full range of bioreactors, from 2ml to 2000L, and adds to the company’s expertise in monoclonal antibody (mAb) process development.

The acquisition expands MilliporeSigma’s portfolio to a full range of bioreactors, from 2ml to 2000L, and adds to the company’s expertise in monoclonal antibody (mAb) process development.

The updated guidance document clarifies what constitutes a statement of the basis for the deficiency and includes examples of well-constructed deficiencies and industry responses to facilitate a more efficient review process.

Bringing design and quality assurance processes together earlier in the device development process can reduce costs and nonconformances, while improving outcomes.

The two new final guidances are intended to increase transparency to stakeholders on the FDA’s approach to the issuance and tracking of 522 orders and post-approval study requirements.

Inspecting for quality after a process is completed is reactive and outdated. Instead, the future lies in predicting quality and quality issues. For medical device manufacturers, the advantages in predictive quality are so great they simply cannot be ignored.

Jordi labs and its team of Ph.D. analytical chemists developed a proprietary, multi-detector approach to ensure that all extractables are accurately characterized to comply with global materials testing regulatory requirements.

“We are excited that organizations like Innovaccer are working to improve trust in clinical data and help ensure the data’s accuracy and broader usability.”
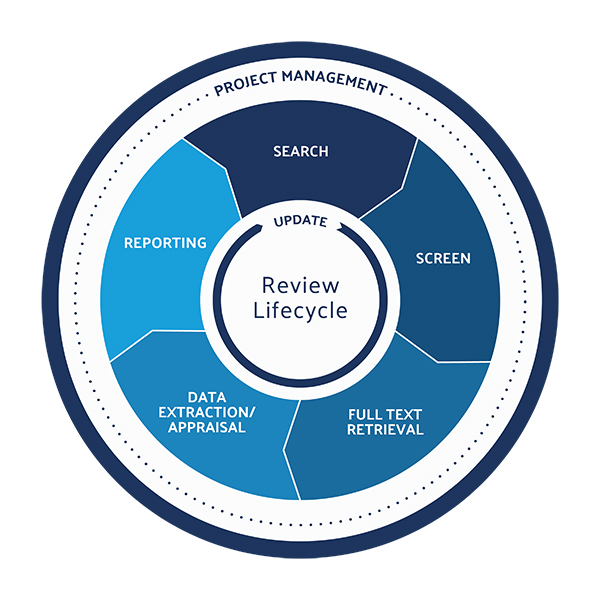
Double-digit growth of the company’s literature review software platform, DistillerSR, spurred $20 million in funding from the growth investment consortium led by Thomvest Ventures.

Companies can make mistakes during drug and device development and in the post-marketing maintenance phase, but all too often, organizations apply a temporary solution to the problem and move on. In this article, Helen Lowe at Arriello addresses how Corrective and Preventive Action (CAPA) management provides a better approach to discover the root cause of the problem.

“The MedAccred Labeling, Printing and UDI Task Group’s role is to provide medical device companies with structure and requirements to assist in identifying and implementing industry best practices for global market compliance.”