

How can drug delivery devices manage competing priorities – reducing overall carbon footprint without expense to patient and practitioner safety or treatment efficacy. Sustainability strategies must account for commercial and budgetary pressures.

How can drug delivery devices manage competing priorities – reducing overall carbon footprint without expense to patient and practitioner safety or treatment efficacy. Sustainability strategies must account for commercial and budgetary pressures.

Because of the environmental and health risks that PFAS ─ per- and polyfluoroalkyl substances ─ pose, they are subject to a potential ban across the European Union. Such a ban would introduce a need for medical device manufacturers to identify and discontinue the sale of products containing PFAS, while also developing safe and effective alternatives. How will suppliers across the EU approach the impending shift away from these “forever chemicals”?
Medical Device reimbursement levels in Japan are now more in line with the reimbursement levels in the EU. The Japanese government has pledged not to allow Japanese device reimbursement to be as high as the US prices.

Medical device companies are aiming to optimize their Clinical Evaluation Report (CER) submissions and streamline compliance process. How are RA teams putting together practical strategies for improving efficiency, reducing risk, and staying compliant with MDR regulations?
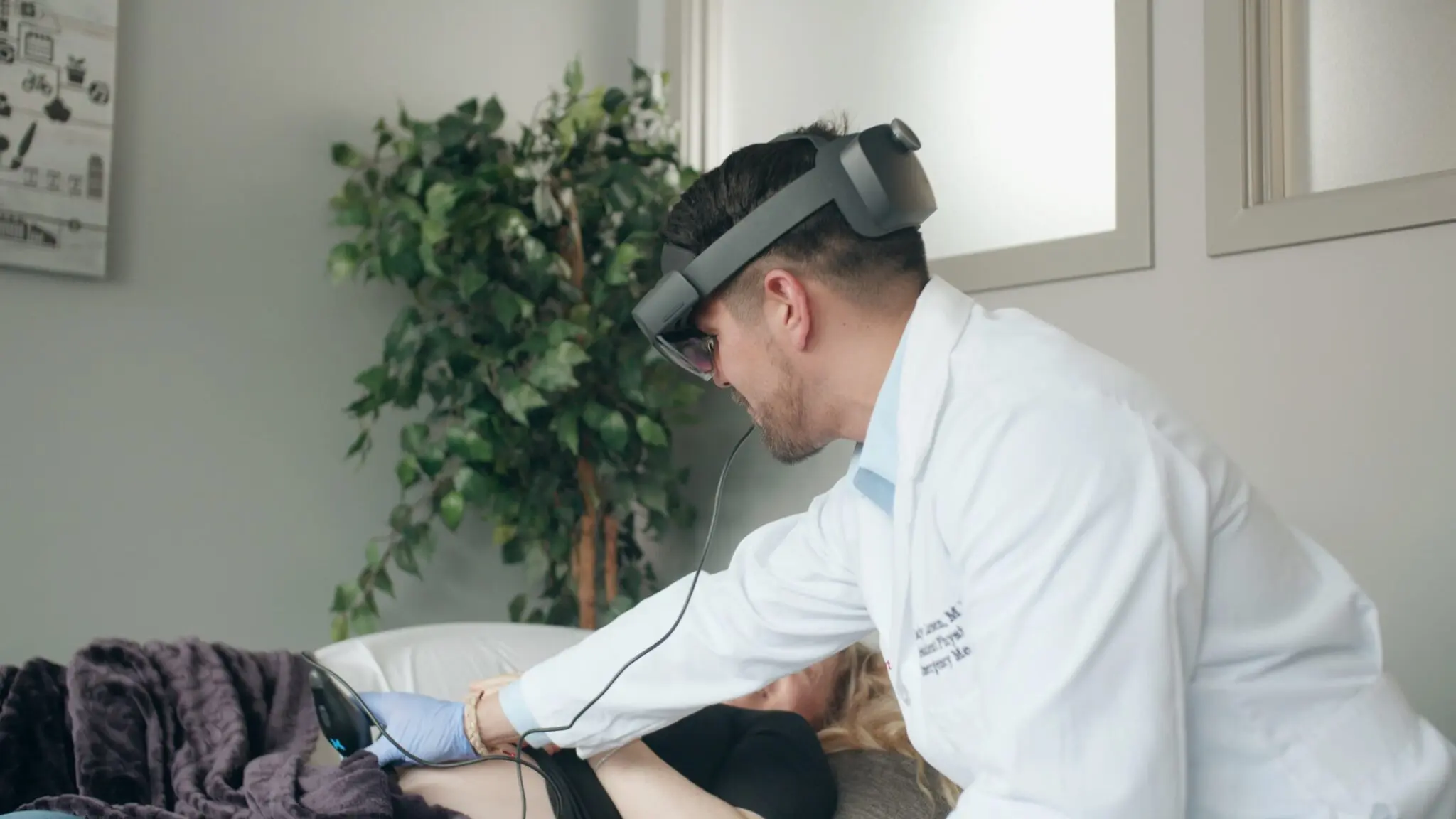
AI has the ability to revolutionize the medical landscape, but to enable this, Quality Engineering is critically important.

If you’re considering a RIM system, it’s critical to determine the right scope, software capabilities, and balance between cost and value. Learn how to evaluate each piece with this RIM buyer’s guide for medtech.

The MDIC Annual Public Forum 2024 kicked off this week with experts from the National Evaluation System for health Technology (NEST), the Centers for Medicare and Medicaid Services (CMS), and the FDA. Topics included the future of real-world evidence (RWE) and the integration of AI into the healthcare ecosystem and how can we leverage emerging technologies to bring innovative and safer solutions to patients.

MedTech organizations need to focus on the human factor of change and achieve top-down alignment, buy-in, accountability, and clear communication along the way.

To streamline processes, enhance transparency, and improve the overall efficiency of conducting clinical trials in the EU/EEA, the 3-year transition period from the CTD to the CTR requirements was established. With the end of the transition period approaching, all ongoing clinical trials that were approved under the CTD will have to be fully be transitioned to comply with the CTR. Failure to comply by 2025 will bring serious consequences.

In the light of new research, Peter Muller and Mike Baird of Schlafender Hase assess how well Class 2 and 3 device manufacturers in Europe and the US are adapting to a rise in regulatory controls.