

The talent behind a company’s solutions is its biggest competitive advantage. For many medtech companies, attracting candidates requires taking a fresh look at the recruiting and retention process.

The talent behind a company’s solutions is its biggest competitive advantage. For many medtech companies, attracting candidates requires taking a fresh look at the recruiting and retention process.

Although opportunities in Japan are still considerable, foreign device companies need to do their homework to be successful.

Digital transformation is especially important to medical device manufacturers because they must have good quality data, and especially tracking metrics, to track complaints and device performance to comply with regulatory requirements. And these requirements are just getting stricter.

Intensive chemistry requirements are creating massive data sets of chemicals, all of which need to be assessed for biological safety. This deluge of data is making equivocal results far more common than ever before. Fortunately, manufacturers can take steps to mitigate risk and support the safety of their products.

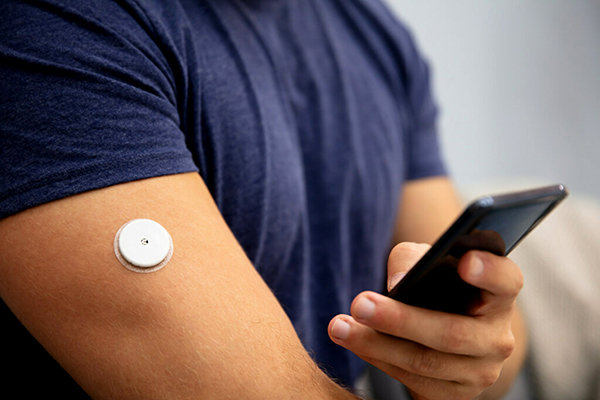
The mHealth industry is rapidly disrupting traditional healthcare delivery by leveraging the power of mobile communication technologies and wearable devices.

This article highlights what manufacturers should prioritize and areas in which new information may still emerge, along with providing a starting point for businesses that need to familiarize themselves with the UK Conformity Assessed marking requirements.

If teams across regulatory, quality and safety functions—as well as further across the life sciences enterprise—are to be able to think and operate in more agile and dynamic ways to achieve what is necessary, then the way that they generate, manage and store documents and data needs to change fundamentally. Regulatory changes (e.g., towards data-driven submissions activity, more dynamic item/label tracking, etc.) are prompting some of this change, but cannot be relied upon exclusively to drive the process improvements now needed.
As an essential material that creates an intimate connection between the device and its user, and is paramount to functionality, the consideration of adhesives should be brought in at the beginning of the design process.

As the country emerges from the COVID-19 pandemic, it’s time to evaluate and assess lessons learned and find any possible silver linings derived from the crisis. This includes telehealth and the use of remote technology.

EMA’s IDMP is not the pinnacle of data-based product/regulatory information management. It is simply the first in what will be a long line of digital requirements to emerge globally, across all facets of life sciences. This article reviews best practices for companies looking to maximize the ROI of their data-driven process transformation initiatives by making them more broadly fit for the future.