

The certification comes at a time when many companies have been saying that the May 2020 deadline is too soon.

The certification comes at a time when many companies have been saying that the May 2020 deadline is too soon.

Will more medical devices be impacted?

For founders and owners of privately held medical product businesses, the amount of private investment dollars available is significant.

It is the pot of gold at the end of the medical rainbow—the promise that, by harnessing data, researchers can tailor drugs, devices, and treatments to the genetic configuration, lifestyle, and environment of individual patients.

Medtech decision makers must accurately predict future trends in order to chart the best future directions and identify the most advantageous areas for business investment.

There are several critical questions that your internal team should be ready to address when preparing product portfolios and seeking out a CRO.
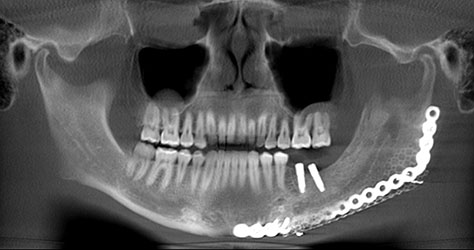
The first steps into clinically practical tissue engineering are through in-situ tissue engineering of bone.

After a series of delays and false starts, EMA is now progressing its ISO IDMP agenda with firm intent. The first implementation guide is out now for consultation, to be followed by a second actionable version by next year. Life sciences companies targeting Europe will have a year to comply with all the measures—but this will be a lot of work.

The agency is encouraging the industry to develop sterilization approaches that either don’t rely on ethylene oxide or reduce emissions from this method.

Understanding the value of the VA and DoD device markets and how to best optimize access and revenue.