

How can drug delivery devices manage competing priorities – reducing overall carbon footprint without expense to patient and practitioner safety or treatment efficacy. Sustainability strategies must account for commercial and budgetary pressures.

How can drug delivery devices manage competing priorities – reducing overall carbon footprint without expense to patient and practitioner safety or treatment efficacy. Sustainability strategies must account for commercial and budgetary pressures.

Diagnostic manufacturers and pharmaceutical sponsors with therapeutics that rely on biomarker identification or companion diagnostics should adjust their commercialization and partnership strategies to fit the new regulatory environment.

Rama Chellappa, PhD, John Hopkins University Bloomberg Distinguished Professor in electrical, computer, and biomedical engineering, and co-author of “Can We Trust AI?” looks at the promise of AI in health care and how we can best utilize this extraordinary tool to save lives and improve health equity.

To avoid delays in timelines, companies should develop IFU cleaning instructions with the worst-case clinical use and contamination method in mind.
The pandemic is helping realize the potential of ISO IDMP data standards in relation to adverse event reporting, electronic prescribing and medicines control in the supply chain.

No longer just “the big company advantage,” Arlen Ward discusses how CM&S is an accepted and viable device industry path to market for large and small medical device makers alike.

Although the full impact of COVID-19 is uncertain, one sure thing is that industry continues to crave information to help them navigate EU MDR.

In general, the Asian markets have controlled the COVID-19 virus successfully outside of China, but its effect has still led to new developments and trends.
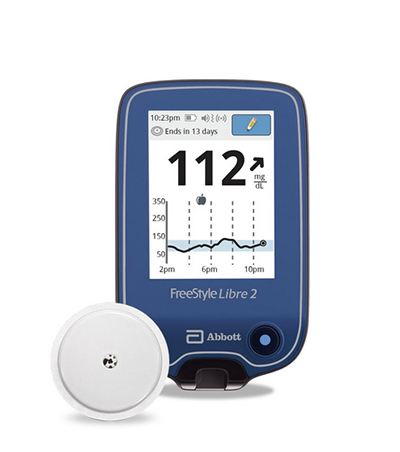
The FreeStyle Libre 2 now has indications for children ages four and older.

The goal of the guidance is to ensure that manufacturers are prepared to report adverse events during a pandemic.