
What’s getting R&D attention? The impact of upcoming regulations, A.I., new cybersecurity risks, developments in battery power and innovation, collaborative efforts, and other impacts on the industry in the year ahead.

What’s getting R&D attention? The impact of upcoming regulations, A.I., new cybersecurity risks, developments in battery power and innovation, collaborative efforts, and other impacts on the industry in the year ahead.

Cardiovascular conditions are the leading cause of death. Fortunately, industry leaders are discovering innovative ways to diagnose and treat them. See how mobile apps and AI will change this field in 2025 and beyond.

“Tariffs may have negative consequences for a continually growing market due to an aging population and the increasing prevalence of long-term illnesses. Companies will be forced to increase prices to make up for losses incurred by the proposed tariffs which may cause supply chain disruptions.”

MTI Viewpoints are insights shared by industry relative to healthcare and the advancement of medical technology.
The AI Training Dataset Market size is projected CAGR of 27.7% in the coming years. By 2024, the market had reached an approximate value of USD 2.82 billion and is forecasted to reach USD 9.58 billion by 2029, according to a new report by MarketsandMarkets™.

Because of the environmental and health risks that PFAS ─ per- and polyfluoroalkyl substances ─ pose, they are subject to a potential ban across the European Union. Such a ban would introduce a need for medical device manufacturers to identify and discontinue the sale of products containing PFAS, while also developing safe and effective alternatives. How will suppliers across the EU approach the impending shift away from these “forever chemicals”?

Harnessing the power of big data from medical device software offers real-time monitoring, predictive analytics, and personalized treatment plans, significantly enhancing the doctor to patient relationship as well as patient outcomes.
J&J MedTech announced today the U.S. launch of the VOLT™ Plating System. The launch of this system represents an evolution in fracture management designed to improve stability, enhance performance, and increase efficiency.
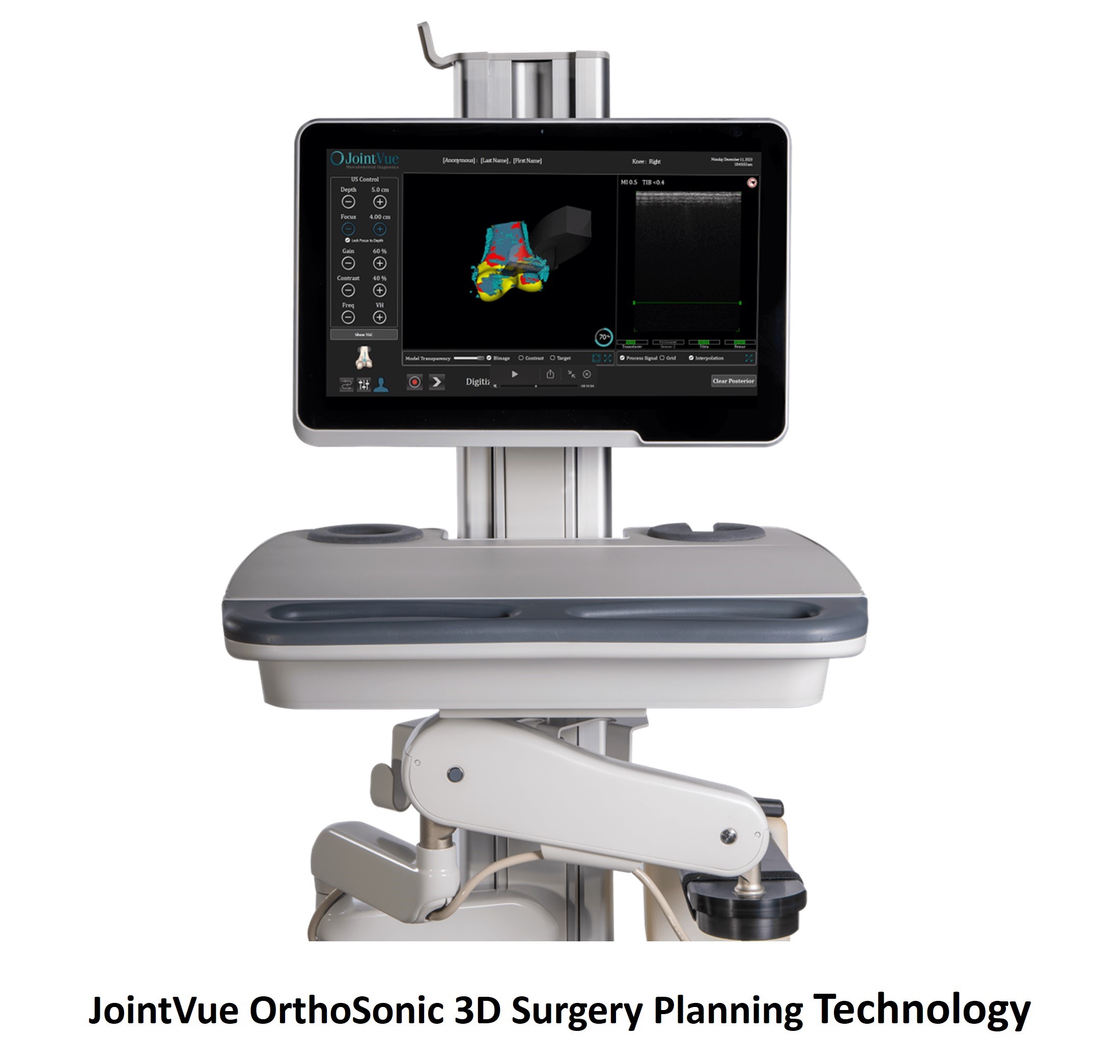
Smith+Nephew and JointVue signed a co-marketing agreement for OrthoSonic 3D surgery planning technology. The technology allows surgeons using the Smith & Nephew Cori surgical robot to create personalized knee arthroplasty procedure plans.

What are the essentials for compliant claims? How do you support and monitor claims in packaging and promotional content? Creating and following internal procedures is a strong defense.