

Collaboration tackles complex challenges using data science to improve decision-making in cancer care.

Collaboration tackles complex challenges using data science to improve decision-making in cancer care.

To streamline processes, enhance transparency, and improve the overall efficiency of conducting clinical trials in the EU/EEA, the 3-year transition period from the CTD to the CTR requirements was established. With the end of the transition period approaching, all ongoing clinical trials that were approved under the CTD will have to be fully be transitioned to comply with the CTR. Failure to comply by 2025 will bring serious consequences.

Following a “alarming” increase in medical device submissions containing unreliable data, the FDA is reminding manufacturers and study sponsors that they are responsible for qualifying third-party test labs and closely scrutinizing all testing data.
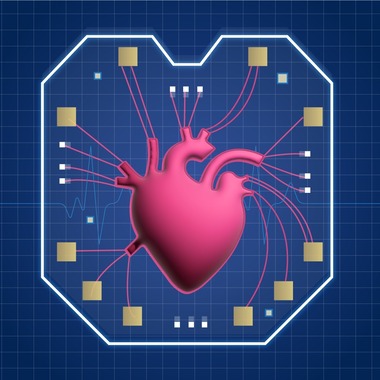
The National Institute of Standards and Technology (NIST) has developed a system that mimics humanlike models for studying cardiovascular disease to reduce the reliance on animal testing and shorten drug development timelines.

Genomics data scientist Harsha Rajasimha, Ph.D., Founder and Executive Chairman of IndoUSrare, highlights the risks of developing AI/ML algorithms based on biased data, as well as efforts underway to improve global collaboration on the collection and sharing of health data that may help us realize the potential of AI in diagnoses and treatment of rare diseases.

AI not only improves data collection and analysis, it impacts which products are engaged in clinical trials, determines necessary medical criteria, helps design the trials and can even choose the best participating facilities. The result is, organizations that leverage AI will be more successful and will go to market faster than those that don’t.

“This acquisition allows us to leverage Courante’s extensive experience in oncology trials and enhance our capabilities in managing global studies.”

The FDA, in collaboration with the Clinical Trials Transformation Initiative (CTTI), is hosting a two-day virtual public workshop on increasing the enrollment of historically underrepresented populations in clinical studies and encouraging clinical study participation that reflects the prevalence of the disease or condition among demographic subgroups. The workshop is scheduled for Wednesday, November 29, and Thursday, November 30, 2023, from 10:00am-2:00pm ET.

Pulvinar Neuro has received a $3 million dollar NIH grant to further its research on noninvasive transcranial alternating current stimulation for the treatment of depression.

Chris Fourment, MD, Senior Vice President, Clinical Strategy at Iterative Health, who specializes in structuring clinical trial programs, discusses the new FDA guidance on decentralized clinical trials, and how DCTs can help spur health and research equity.