PRESS RELEASE: MTI Staff was not involved in writing this content
Next-Generation Plating System combines flexibility and stability for advanced fracture care1-4* across orthopaedic procedures5,6
[MTI Newswire] WEST CHESTER, Penn. – October 10, 2024 – Johnson & Johnson MedTech, a global leader in orthopaedic technologies and solutions, announced the first phase of the VOLT™ Variable Angle Optimized Locking Technology Plating System launch. The innovative system represents an evolution in our fracture management solutions designed to improve stability, enhance performance and increase efficiency.†
Orthopaedic trauma remains a serious concern worldwide, with the U.S. alone experiencing 40 million emergencies annually, many involving complex fractures that require open reduction and internal fixation with plates and screws. The need for improved solutions to address these serious injuries is critical.7
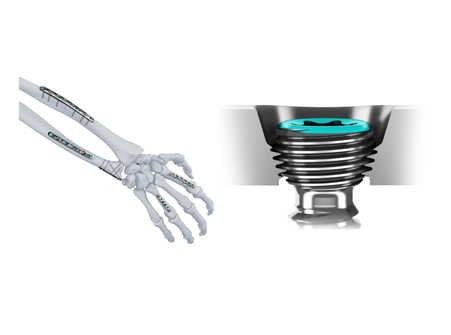
In addition to the innovative locking technology, the VOLT™ Plating System offers an expanded range of plate shapes, lengths, and screw lengths to accommodate different fracture reduction and fixation needs.8¶ The implants are designed to minimize prominence to reduce the risk of soft tissue irritation.9|| The system also features a versatile tray design that allows for multiple set configurations based on clinical requirements, and color-coded instruments simplify identification with the goal of improved surgical workflow efficiency for operating room staff.10
“The launch of the VOLT™ Plating System underscores our leadership in trauma care and marks the next chapter in our long-standing commitment to innovation,” said Aldo Denti, company group chair, orthopaedics, Johnson & Johnson MedTech. “For over 50 years, we’ve been driving advancements in fracture management, and this system exemplifies our dedication to providing surgeons with the strongest, most comprehensive and versatile solutions to address the varied needs and achieve the best possible patient outcomes.”
The system has been designed with input from a global team of expert surgeons from around the world. Michael Raschke*, M.D., Chairperson AO Technical Commission Executive Board (TCEB) and AO Technical Commission Trauma (AO TC Trauma)††, noted: “The VOLT™ system is a huge accomplishment. It’s a comprehensive system that combines well-established AO principles with state-of-the-art functionality. I believe it will quickly become the standard of care in treating skeletal fractures.”
Dr. Christoph Sommer*, M.D., Chairperson Lower Extremity Global Expert Committee (LEGEC) and Next Generation Plating Task Force (NGPTF), added: “Everything about the new system has been improved. More options, greatly simplified instrumentation, and the ability to place screws at different angles are all significant advancements. I’m excited to begin using the system and confident it will be well received by surgeons and staff.”
Engineered with precision threaded locking, the VOLT™ Plating System offers enhanced construct stability.1-4‡ Unlike competitive systems that rely on screw threads to cut into the plates for locking, the VOLT™ mechanism utilizes tightly toleranced screw hole threads to improve plate-to-screw engagement.1-4‡ This advanced design provides the stability of the Locking Compression Plating™ while allowing for flexible variable angles.1-4* It will be available in both stainless steel and titanium.5,6
Following clearance from the United States Food and Drug Administration (FDA), the VOLT™ Mini and Small Fragment Plating Systems are now available for commercial use. The VOLT™ Distal Radius and VOLT™ Proximal Humerus Plating Systems will be introduced in 2025 with additional anatomic plating solutions to follow.
The Plating System will be featured at the upcoming Orthopaedic Trauma Association (OTA) meeting happening October 23-26 in Montreal, Canada, where it will be showcased alongside the latest innovations in trauma technology. Attendees are invited to visit Johnson & Johnson MedTech at Booth #369 to explore the system and join the industry session, “Clinical Evolution of Mini Fragment Plating,” taking place on Friday, October 25th, from 12:45 to 1:35 p.m. EDT in Exhibit Hall – Theatre C to learn more.
About Johnson & Johnson
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow, and profoundly impact health for humanity. Learn more about our MedTech sector’s global scale and deep expertise in cardiovascular, orthopedics, surgery and vision solutions at https://thenext.jnjmedtech.com. Follow us at @JNJMedTech and on LinkedIn. DePuy Synthes Products, Inc. is a Johnson & Johnson MedTech company.
Footnotes
* Cantilever testing done comparing VOLT Mini and Small Fragment Systems to VA LCP Plating System, and LCP Plating System
**Drs. Raschke and Sommer are members of the AO Technical Commission (AO TC).
†Important Information: Prior to use, refer to the instructions for use supplied with the device(s) for indications, contraindications, side effects, warnings and precautions.
††AO Foundation is a 3rd party medically guided, not-for-profit organization led by an international group of surgeons specialized in the treatment of trauma and disorders of the musculoskeletal system.
¶Compared to DePuy Synthes Modular Mini Fragment LCP System, Smith and Nephew Evos Mini Plating System, and Stryker VariAx 2 Mini Fragment System.
||Engineering Rationale comparing 2.0 mm, 2.4 mm, and 2.7 mm VOLT Straight and Adaption Plates with respective screws to 2.0 mm, 2.4 mm and 2.7 mm Modular Mini LCP Straight and Adaption Plates with respective screws, cortex screws placed in combi-hole at nominal angle.
‡Cantilever testing done comparing VOLT to Smith and Nephew Evos Mini and Small Plating System, and Stryker VariAx2 Mini and Small Fragment Plating Systems.
The third-party trademarks used herein are the trademarks of their respective owners.
Important Information: Prior to use, refer to the instructions for use supplied with the device(s) or indications, contraindications, warnings and precautions.
1 DePuy Synthes VOLT 2.0 – Marketing Claims Interface Testing. September 4, 2024. Windchill # 501969571
2 DePuy Synthes VOLT 2.4 – Marketing Claims Interface Testing. September 4, 2024. Windchill # 502025403
3 DePuy Synthes VOLT 2.7 – Marketing Claims Interface Testing. September 4, 2024. Windchill # 502025404
4 DePuy Synthes VOLT 3.5 – Marketing Claims Interface Testing. September 2, 2024. Windchill #501969506
5 VOLT™ Small Fragment Plating System Instructions for Use GP3149. DePuy Synthes.
6 VOLT™ Mini Fragment Plating System. Instructions for Use GP3146. DePuy Synthes.
7 FastStats – emergency department visits. Centers for Disease Control and Prevention. April 15, 2024. Accessed October 3, 2024. https://www.cdc.gov/nchs/fastats/emergency-department.htm.
8 DePuy Synthes. Benchmark Analysis: VOLT Mini and Small Fragment Plating System Comprehensive Implant Portfolio. 06_19_2024. Windchill #501988740
9 DePuy Synthes. VOLT Mini Fragment Engineering Analysis, screw prominence, construct height and 15 deg screw clearance. 06_27_2024. Windchill #501983787
10 DePuy Synthes Color- Coding, Modularity, and Instrumentation Ease of Use Report. September 12, 2024. Windchill #502069822


