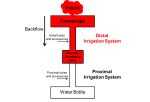
When undergoing procedures that use a flexible gastrointestinal endoscope, the risk of cross-contamination between patients is a concern—significant enough that today FDA released a guidance document that details how manufacturers can mitigate the risk. Specifically of interest are the components—the irrigation valves and accessories—that are used with the endoscopes. Clinicians many times use a water bottle to provide irrigation during a colonoscopy or esophagogastroduodenoscopy. The problem, according to FDA, is that this water bottle is used on multiple patients without being reprocessed between use and as a result, the tubing and connectors can be contaminated with blood, stool, and other patient fluids due to backflow (when the fluids travel between the endoscope channels). All devices should have a backflow-prevention mechanism.
FDA’s guidance document, Mitigating the Risk of Cross-Contamination from Valves and Accessories Used for Irrigation Through Flexible Gastrointestinal Endoscopes, defines the components of flexible gastrointestinal endoscopes, along with the accessories. The draft guidance was issued January 20, 2015.
The document makes recommendations on the device design to prevent backflow, including performance testing protocol, outlines practices involving the reprocessing or disposal of the irrigation system, and details how manufacturers should label the device to ensure proper use.
“Backflow-Prevention Valve: The valve that is intended to prevent the proximal irrigation system from being contaminated by backflow of fluids from the patient. When multiple valves are present in the irrigation system, the backflow-prevention valve is the one closest to the patient. When used in the auxiliary-water/forward-water-jet channel, this valve is frequently referred to as a ‘one-way valve.’” – FDA
If the irrigation system does not have a backflow-prevention valve (or other contamination prevention mechanism), “all components of the irrigation system should be reprocessed or discarded after being used during a procedure in one patient and before starting one in the next patient,” according to the FDA guidance document.