Non-Fusion Alternative a Promising Treatment for Adolescent Scoliosis
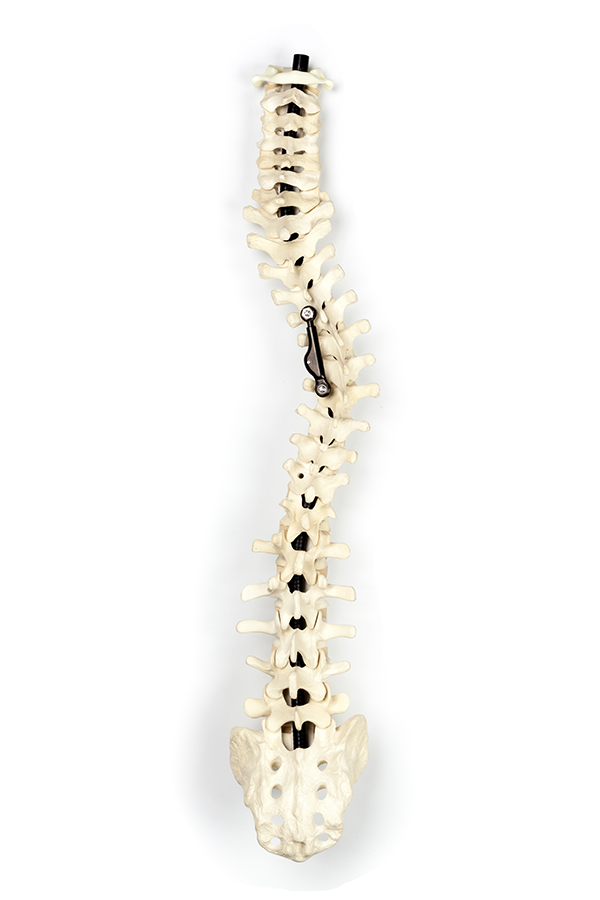
Adolescent idiopathic scoliosis (AIS), a condition that causes the spine to curve sideways, is stressful and debilitating, hindering the activity and well being of tens of thousands of children worldwide. AIS affects about 2% of the pediatric population, with 1 in 1000 cases requiring surgery (surgery is recommended when the spinal curve progresses such that it needs correction, which is generally at 40 to 60 degrees), according to Ted Bird, chairman of the board at Israel-based ApiFix, Ltd., a company developing technology to treat AIS. Bird adds that globally, the number of children who have spinal curves that require surgery approaches 40,000; about 7500 of those children live in the United States. The condition is more prevalent in teenage girls.
Spinal fusion is the current gold standard for treating AIS. Considered major surgery, the procedure can be fairly traumatic for children, as it can involve the permanent fusion of 10 to 12 vertebrae, resulting in a reduced rate of mobility, which is particularly concerning in a population that is still physically developing. In addition, the fused parts of the spine stop developing altogether.
ApiFix recently received $25,000 from the National Capital Consortium for Pediatric Device Innovation (NCC-PDI) for its corrective minimally invasive system (also called ApiFix) for AIS. As a non-fusion alternative, the technology preserves spinal mobility and can make a significant difference in patient lives, as they spend less time in the hospital and experience a faster rate of recovery. “It’s dramatically different and new in a field that hasn’t changed in 30 years,” says Bird, who spoke with MedTech Intelligence about the development of the ApiFix system and the company’s plans to bring the technology to the U.S. market within the next year.
MedTech Intelligence: What was the company’s process for developing a system that specifically helps the pediatric population?
Ted Bird: It needed to be a system that accommodates for growth. In younger kids, if you’re putting in a metal fixture to correct a curve, they have go through multiple corrections of the implanted hardware to allow it to expand and grow. It’s a process for patients and their families, and it’s expensive for the system to keep redoing procedures to adjust the fusion mechanism. Those are today’s standards.
ApiFix involves one minimally invasive surgery that takes less than one hour. The implanted construct is placed at the apex of the major curve that’s being corrected: Two screws [are implanted] in a traditional way (to stabilize the spine), along with a rod that has an internal ratchet mechanism to enable gradual post operative elongation of the device after surgery. After the patient has recuperated from the minimally invasive surgery, the device is activated through specific spinal exercises that the patient performs post operatively. The ratchet enables growth and creates the curve correction.
| Surgical Time (Hours) | Incision Size (cm) | Time Hospital (Days) | |
| ApiFix System | 1 | 10–12 | 2–3 |
| Spinal Fusion (Traditional) |
Up to 6 | 45–50 | 6–7 |
There were some considerations that went into the design criteria for this system: [First], ensuring that it is strong enough and will work; secondly, that it’s going to hold the correction of the curve and that the ratcheting mechanism would not slip back. It also had to be made of a material that doesn’t create any wear debris, since it’s a medical implant.
MTI: With the technology development occurring in Israel, what is the company’s strategy to market?
Bird: [ApiFix] founders Yizhar Floman, M.D., and Uri Arnin (the CEO) have strong backgrounds in spine. They started development and received a grant from the Office of the Chief Scientist, which provides funding for many Israeli start-ups. They were housed and supported by an incubator in northern Israel called Trendlines Medical [http://trendlines.com/medical/], through which they raised additional money, and were primarily supported by private U.S. investors to allow them to finish the prototype and the patent work and to get to a first-in-man clinical trial in Israel; then [they] applied for CE Mark approval.
The beauty in it is the simplicity. It’s the simple mechanical ratcheting mechanism that is activated by the patient who is involved in the process afterwards. – Ted Bird, chairman of the board, ApiFix
So within a year of getting started in 2011, they were able to successfully complete a first-in-man trial and, along a parallel path, apply for and receive a CE Mark within 18 months.
The company has carefully selected the clinical sites that have access to the technology in order to control the clinical trial with the system. They started in late 2012 and now have more than 70 patients [enrolled] in six European countries and Israel. At least six to seven of the patients are now three years post operative; 24 are over two years post operative; almost 40 are over one-year post operative; all with no device or screw breakage. The goal of the study is to show that this system works to reduce the scoliosis curves to below 35 degrees. Those pinpoints have been met so far, and with excellent results.
Now we’re in the process of applying for FDA approval to introduce the system in the United States through a humanitarian device exemption (HDE) approach.
MTI: How will the company use the $25,000 award from NCC-PDI?
Bird: The money will be used for the various tests that FDA requires (biomechanical tests) for the HDE submission, which we’ll complete and submit later this year. The timing was perfect because we already started some of them. We’re concurrently looking to raise our B round of financing to fully complete the FDA testing, expand our distribution in Europe and prepare for U.S. introduction, which we expect to happen sometime in late 2017.
MTI: What advice can you offer companies that are developing technologies for the pediatric market?
Bird: Very important with any medical device, particularly in this population, is to seek advice from leaders in the field. ApiFix was fortunate to have the idea come from a very experienced pediatric orthopedic spinal deformity surgeon. Through Professor Floman’s connections, we were able to get feedback from other pediatric deformity specialist surgeons about the design and approach, and the exercises for patients, which was very important. You can’t under estimate the importance of getting that feedback as early in the process as possible.
Secondly, when you have a disruptive and novel technology—be careful not to roll out or expand the distribution or availability of the technology until you’ve clearly proven and validated that it works well. ApiFix has done a very good job of carefully and responsibly controlling the release—even with [obtaining] a CE Mark and not being tempted to expand too quickly and possibly receive less-than-optimal outcomes by not carefully selecting the right sites. Make sure that those using the technology are using it for the right indications and not expanding indications too widely or using it on the wrong patients. It’s important in the pediatric population, but particularly with any new innovative technology—don’t kill it before it gets a chance to shine.