Abiomed Updates IFU for Impella Blood Pumps Due to Perforation Risks
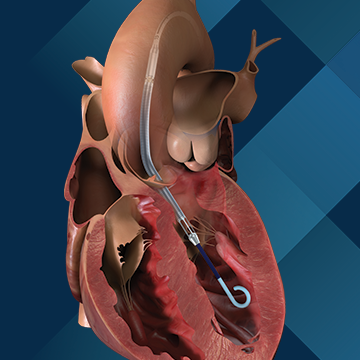
The FDA announced that Abiomed is updating the instructions for use (IFU) of its Impella Left Sided Blood Pumps because the pump catheter may perforate the wall of the left ventricle in the heart. During operations, the Impella device could cut through the wall of the left ventricle. To date, there have been 129 reported serious injuries, including 49 reports of death.
Product names of the affected devices include: Impella 2.5; Impella CP; Impella CP with SmartAssist; Impella 5.0; Impella 5.5 with SmartAssist; and Impella LD.
Use of the affected Impella pumps may cause serious adverse health consequences, including left ventricle perforation or free wall rupture, hypertension, lack of blood flow, and death. IFU has been updated to include warnings about the risk of the inlet perforating through the myocardial wall of the left ventricle due to operator handling.
On December 27, 2023, Abiomed sent all affected customers an Urgent Medical Device Correction letter. The letter requested customers to adhere to new and revised warnings:
- Carefully position the pump catheter during operative procedures
- Use imaging when advancing or torquing the pump catheter
- Use special care when inserting the pump catheter in patients with certain high risk conditions or during active CPR
- Review the updated warnings in the device Instructions for Use
- Notify everyone at your facility who needs to be informed of this recall correction
- Notify any other facilities where the products have been forwarded of the updated Instructions for Use