Novel Long-Term Hermetic Ultrathin Implant Packaging Technology Based on Ceramic Nanolayers
To realize maximum miniaturization of active implants, the conventional encapsulation/packaging technology for implants based on a titanium or glass package, needs to be replaced. The combination of thin biocompatible polymer films and high-quality ceramic nanolayers proves to fulfill the hermeticity needs of long-term implantable medical devices.
Conventional Packaging Strategy for Active Implants
The interest in active medical implants is continuously growing. Active implants realize a higher degree of supporting functionality: They modify their activity depending on changing circumstances and the needs of the patient. An example is a pacemaker that monitors the patient’s heartbeat and only interferes when needed, taking into account the patient’s activity level. For an active implant to achieve this type of smart reaction, it must contain various moisture sensitive components such as electronics, telemetry, an antenna and a power source. Since direct exposure to body fluids will certainly damage these components, protection is needed. Furthermore, the electronic components themselves are not biocompatible, so the body needs to be protected to avoid leaching of these non-biocompatible substances. Therefore, active implants need to be encapsulated by a superior bi-directional diffusion barrier: No bodily fluids or ions should diffuse into the implant, and no implant materials should leach into the local tissue. Placing components in a titanium (Ti) box is a common practice that achieves proper encapsulation, but it has significant drawbacks. While it is a well-proven hermetic implant package, the Ti-box is rather large and rigid, which evokes a pronounced foreign body reaction (FBR) upon implantation, resulting in a thick fibrous tissue encapsulation, which might decrease the implant’s sensor sensitivity. Furthermore, mechanical mismatch of the Ti-box and local tissue might cause chronic discomfort for the patient.
Smaller Active Implants Need a Thin Hermetic Encapsulation Technology
A smaller, thinner and soft implant encapsulation technology has significant advantages. The implantation procedure might be much less invasive, resulting in a short hospital stay, faster patient recovery, and fewer infection risks. The FBR will be less pronounced, and the risk of chronic patient discomfort is much less due to the reduced implant size and enhanced mechanical match with the soft local tissue. Finally, if a thin, flexible encapsulation is used, the device—or at least its sensoric part— can be made very small, enabling insertion into tiny and sensitive body areas that can never be reached by a sensor using a conventional Ti-box. Examples include electrodes of nerve stimulating devices, insertion into a nerve bundle with limited nerve damage or flexible retinal implants in the eye.
Ultrathin Barriers with Extreme Hermeticity
Thin layers of biocompatible and biostable polymers such as medical-grade polyimide, parylene, and PDMS can be applied to establish an alternative thin film encapsulation strategy. Although these materials are known as moisture barriers, they will never provide sufficient hermeticity to active (long -term) implants.1,2 Fortunately, combining polymers with ultrathin layers of selected ceramic materials, such as Al2O3 or HfO2, will significantly enhance the barrier function of polymers.3 These ceramics are biocompatible, and as long as the deposition is of very high quality, they have superior diffusion barrier properties and even as ultrathin films. Atomic layer deposition (ALD) techniques allow for the deposition of ceramics in a slow but extremely accurate manner, where each atom is deposited under tight process control to avoid errors in the material structure. High-class cleanroom conditions are essential to ensure there are no particles included in the ALD barriers. Detailed process optimization is performed to allow for high-quality deposition at temperatures varying from 250°C and lower, as low as 100°C, which enables ALD deposition on polymers.
The combination of 1 polymer layer with 1 ALD layer/stack is called a dyad. Though a small amount of moisture can penetrate the polymer barrier layer, the ceramic barrier stops it, but the very scarce defects still present in the ALD results in the local penetration of a minimal amount of moisture, thus highlighting the importance of high-quality deposition of the ALD ceramics.4 If an active implant is going to be present in the body for decades, there must be no risk taken regarding encapsulation hermeticity. Hence extreme barriers are fabricated by stacking several dyads (see Figure 1). It is essential to use high-quality ALD ceramics as they provide superior adhesion between all layers of the total encapsulation.5 The resulting moisture diffusion pad is very tiny and long. Hence the moisture penetration is reduced to almost zero, ensuring superior hermeticity.
In order to fabricate such superior moisture barriers, high-quality ALD processes are optimized for Al2O3 and HfO2 in a Savannah tool from Veeco (Ultratech) at temperatures varying between 100°C and 250°C, enabling the deposition of ceramics on various polymers with different thermal budgets. Lower temperature ceramics still have excellent properties, but their deposition speed becomes very low. On the bright side, only a few tens of nanometers of the ceramic materials are sufficient to ensure superior hermeticity. Al2O3 is regarded as an excellent diffusion barrier but hydrolyzes in water, which deteriorates the barrier quality.6 Therefore, Al2O3 is sandwiched between HfO2 layers, a ceramic which is very stable in water. The obtained HfO2/Al2O3/HfO2 stack is further called ALD-3. An ALD-3 diffusion barrier is developed consisting of 8/20/8nm HfO2/Al2O3/HfO2 deposited at 250°C for polyimide-based implant encapsulations, as well as a barrier of 15/20/15nm HfO2/Al2O3/HfO2 deposited at 120°C or 150°C, to be combined with Parylene F-VT4. Water Vapor Transition Rate (WVTR) measurements and copper corrosion tests, performed at 37°C or under accelerated conditions (higher temperature), are used to evaluate these barriers.
WVTR Results
It is essential to realize that WVTR testing is challenging for extreme barriers that have WVTR values well below 10-3 g/m2.day. Many labs do not have sufficiently sensitive equipment, resulting in incorrect WVTR results of extreme barriers. Furthermore, the lag time to reach the steady-state situation in extreme barriers can take many months or even over one year! Hence many scientists do not wait for steady-state conditions, often resulting in the wrong (too low) WVTR values.4,7 Two barriers were tested at 37°C and 100% relative humidity (RH): Polyimide PI2611/ALD-3 deposited at 250°C, and parylene F-VT4/ALD-3 deposited at 150°C. Using the HibarSens tool from Sempa allows for WVTR measurements even below 10-5 g/m2.day. Measurements were performed sufficiently long to assure steady-state conditions, realized by preconditioningthe test films at 37°C and 100% RH. The polyimide/ALD-3 barrier shows a very low WVTR of 2.1 10-5g/m2.day (see Figure 2), which is 10,000 times lower compared to polyimide without ALD-3. The Parylen/ALD-3 barrier resulted in a WVTR of 1.3 10-4 g/m2.day, which is a remarkable improvement compared to pure parylene. Although the parylene/ALD-3 barrier has five times higher WVTR, it is still very useful since –in contrary to the polyimide/ALD-3 barrier- it can be applied on devices with significant topography and it is compatible with components with a limited temperature budget. For both barriers, the extreme diffusion stopping power by ultrathin ceramic layers is well proven.
Films with only one dyad were used to reduce the WVTR test duration; however more dyads are certainly preferable regarding hermeticity for later practical implant applications. An attempted test on a stack of three dyads of Polyimide/ALD-3 deposited at 250°C only measured the WVTR at 85°C since the lag time was too long at lower temperatures. The obtained WVTR at 85°C was 4.8 10-4 g/m2.day, which is a factor of three lower than the WVTR of a single dyad of the same materials tested at 85°C. Based on these results, one can estimate that a three dyad stack of polyimide/ALD-3 will have a WVTR between 10–5 and 10–6 g/m2.day at 37°C, which matches perfectly with the hermeticity needs for long-term implants.
Copper Corrosion Tests
Covering Cu patterns of 1 um thick and 30 to 50 µm wide with the same polyimide/ALD-3/Polyimide stack used for the WVTR test described above, allows for testing the barriers more practically. Similar structures without ALD-3 were also fabricated as a reference. Both structures were submersed in saline at 60°C, while the pattern resistivity was measured to detect Cu corrosion due to barrier failure. After six weeks, the structures without ALD-3 showed polymer blistering, and around six months, all Cu was corroded. The Polyimide/ALD-3 stack protected the structures exceptionally well, with no signs of deterioration observed after more than three years of immersion in saline at 60°C (see Figure 3), corresponding to a soaking time of more than 15 years at 37°C. Testing will be continued until meander corrosion is observed.
Polyimide/ALD-3 Barrier Application: Ultrathin Encapsulation for an Active Neural Probe
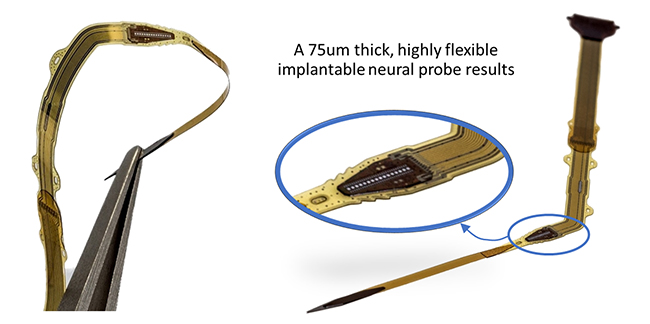
In the frame of the Impress project, executed in collaboration with the University of Florida and funded by DARPA (under auspices of Dr. D. Weber through SPAWAR, Pacific Grant/Contract No. N66001-15-C-4018), an active probe was fabricated based on a CMOS chip carrying 80 electrodes for both stimulation and recording of local neural cells (see Figure 4).
After chip thinning and full encapsulation, the total device is only 75 µm thick, comparable to a thin human hair. The device is also flexible, and combined with a dedicated miniaturized insertion piece, is optimized for intrafascicular insertion in a peripheral nerve with minimum tissue damage (details about fabrication and evaluation of this active probe are demonstrated in numbers 8 and 9 in the References section). A cross-section of the chip, and its encapsulation shows four polyimide/ALD-3 dyads on top and three dyads underneath the chip (see Figure 5). This encapsulated chip was soaked in saline at 60°C for 65 days, showing no signs of corrosion, which corresponds to an excellent hermeticity for about 325 days at 37°C. Additional testing is scheduled.
Conclusion
Researchers have developed a novel implant encapsulation technology by combining ultrathin ALD ceramics with biocompatible polymer thin films and fabricating an extreme hermetic barrier, thus enabling the realization of very thin, flexible, long term hermetic active implants.
References
- Teo A.J.T, et al. (2016). Polymeric Biomaterials for Medical Implants and Devices ACS Biomater. Sci. Eng. 2, 454–472.
- Qin, Y., et al. (2014). Polymer integration for packaging of implantable sensors. Sensors Actuators. B 202 , 758–778.
- Kim, L.H., et al. (2017). Highly-impermeable Al2O3/HfO2 moisture barrier films grown by low-temperature plasma-enhanced atomic layer deposition. Org. Electron. 50, 296–303.
- Graff, G.L., Williford, R.E., and Burrows, P.E. (2004). Mechanisms of vapor permeation through multilayer barrier films: Lag time versus equilibrium permeation. J. Appl. Phys. 96, 1840; https://doi.org/10.1063/1.1768610.
- Schaubroeck, D. et al. (2017). Polyimide-ALD polyimide layers as hermetic encapsulant for implants. XXXI Int. Conf. on Surface Modification Technologies (SMT31).
- Nehm, F. et al. (2015). Breakdown and Protection of ALD Moisture Barrier Thin Films ACS. Appl. Mater. Interfaces. 7, 22121–22127.
- Kiese, S., et al. (2019). Time-dependent water vapor permeation through multilayer barrier films: Empirical versus theoretical results. Thin solid films. 672 , 199-205.
- Op de Beeck, M., et al. (2017). Ultrathin biocompatible implantable chip for bidirectional communication with peripheral nerves. IEEE Biomedical Circuits and Systems Conference (BioCAS); DOI: 10.1109/BIOCAS.2017.8325206.
- Verplancke, et al. (2020). Development of an active high-density transverse intrafascicular microelectrode (hd-TIME) probe. Journal of Micromechanics and Microengineering. 30, 015010.