How to Create Effective Relationships with Your Development Partner
A study conducted by Grand View Research last year projects global growth of the medical device outsourcing market to hit $50.37 billion by 2020; OEMs can thank outsourcing for an estimated 15% reduction in production costs. However, adding these outside design, engineering and contract manufacturing partners can increase the complexity of the product development process. This article discusses the approach companies can take to set expectations and streamline these partnerships.
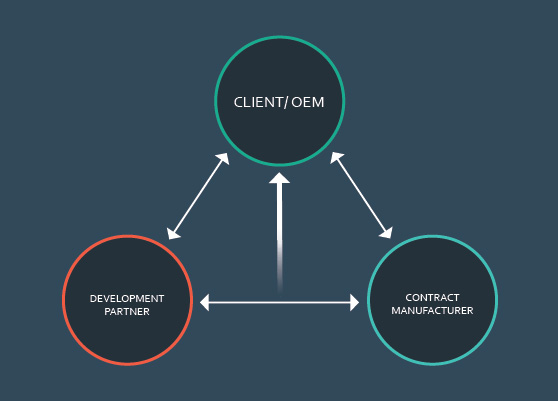
Define and Assign Roles
Each product development team member plays either a key or supporting role at any given point in the process. It is important to clearly define each member’s role as well as take the following steps:
- Set support expectations for both contract manufacturers (CMs) and component suppliers early
- Define who will assemble prototypes within each design phase
- Define which development partner will conduct testing and at what point
- Define who qualifies suppliers (many larger OEMs do this internally, whereas some smaller companies get recommendations from development partners or the CM, who generally have approved vendor lists)
- Define who inspects prototype parts in advance
Continuous Vigilance Equals Fewer Surprises
Effective communication among team members can prevent unexpected issues from negatively affecting the development schedule. The key is to strike a balance between a lack of communication and “over-communication”, which can reduce productivity. Maintaining vigilance in the following areas can help avoid surprises:
- Program management. Diligent project planning should be done by all partners—the project leader is not solely responsible for this task, nor is the client.
- Establish and maintain a master schedule. There are effective tools for generating a detailed project plan (e.g., Microsoft Project), while smaller projects can use less formal planning tools, such as a spreadsheet. The master schedule should be available to all partners involved, with the capability for team members to make quick updates. However, do not give too many members the ability to edit the document, as this can complicate the plan and cause confusion among partners.
- Generate and maintain a cross-functional, open issues tracker. Especially critical for larger projects and independent teams, the tracker should focus on system-level issues as each partner is focusing on issues that pertain to their core responsibility.
- Prepare for the unexpected to mitigate delays. When a product suddenly requires significant technological invention during development, experienced development managers know there will be potential project delays and additional work. However, unexpected delays generally come from major shifts in design direction, with the two specific offenders being feature creep and risk.
- Feature creep. OEMs and start-ups are often blamed for adding new requirements during development, but each team can be guilty of this in the name of an improved product, reduced cost, etc. It is the job of a program manager to keep feature creep under control.
- Risk. The team should generate and maintain a risk tracker that includes technical and program-related risks. Establishing an effective risk tracker ensures that challenges to the program will be addressed as early as possible and that upper management is aware of the risks for tactical decision-making and contingency planning purposes.
Mutual Partner Support
All partnerships require that every member understand how each partner can support the other. While dependent upon the level of each partner’s expertise, if all partners have exceptional experience, it is important to establish this understanding at the beginning of the program.
Minimizing product cost is a team effort. The contract manufacturer is primarily responsible for determining product cost, but the actions and demands of all partners can have a direct impact. The client should calculate and provide a product’s target cost to the development partner as early as possible. In cases where a smaller OEM or startups lacks expertise in determining cost, enlist the help of a development partner that has a strong background in bringing products to market.
Design for manufacturability and assembly (DFMA) to reduce cost. The development partner is responsible for product design, and including the CM in the conversation can help the team select the most appropriate manufacturing process. The CM can also supply process guidelines to help the development partner complete the design.
Stretching manufacturing technologies. Most new products can still be made using standard components and manufacturing processes and industry-standard tolerances. More complex products may require teams to push design and manufacturing beyond their standard limits (i.e., requiring tight tolerances or integrating a new technology). This is yet another reason why it is important to collaborate with the development partner and CM early in the development cycle.
High production volumes. Automated assembly by the CM can provide a significant benefit to high-volume production. This process can involve vision systems, robotic handling, or the addition of special features to individual parts, such as molded-in fiducials for the vision system or features that a robot can grab. Such details will require close collaboration between the developer and the CM.
Effective development teams apply the lessons learned from past experiences to minimize issues in subsequent projects. Concept-to-product realization projects that involve partners who communicate and collaborate effectively are much more likely to result in products that meet or exceed an OEM’s goals. OEMs that both understand how their partners work with each other and are proactive about engaged planning with them create the core of an effective relationship. In this era of multiple outsourced partners, project success is all about experience, communication and planning. In the words of Benjamin Franklin, “If you fail to plan, you are planning to fail.”