How to Achieve Quality through Process Validation
With any manufacturing process, you want certainty in the quality of your products. As you scale production from hundreds or thousands into the millions, you want to know with a high level of confidence that your process produces good parts with consistency. You might start making good parts only to get surprised later by nonconformities as you grow. Those nonconformities occur because of lower quality and higher risk than acceptable.
Process validation helps you improve quality and reduce risks in your manufacturing process. It enables a successful manufacturing process that makes produces quality parts and reduces surprises along the way.
What Is Quality?
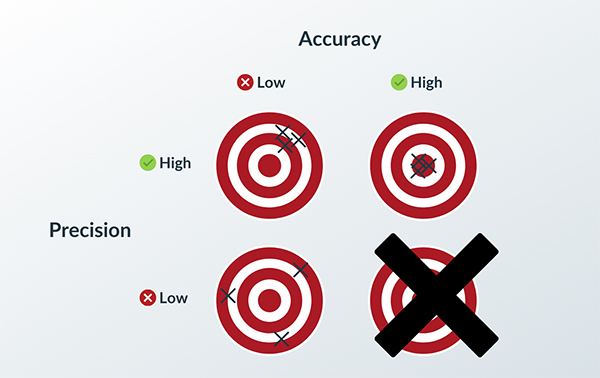
Quality can be defined as low variability of accuracy and precision. Precision reflects how well you hit the same mark, while accuracy reflects the correctness of your mark (see Figure 1).
You can be accurate without being precise and vice versa. A weather forecaster could call for precipitation on Earth next month, which would be accurate but not precise. They could predict 5.3 inches of rain at 39°50′N, 98°35′W on 01/01/2035 at 12:01 AM CT, which would be precise but probably not accurate. A plastic injection molding engineer can predict they will hit a tolerance of +/- 10 microns while the nominal dimension might vary by 40 microns, which would achieve precision but not accuracy.
How Do You Achieve Quality?
Accuracy and precision will experience some variation in any situation. To achieve quality you want to distinguish between special causes of variation and common causes of variation. The order here matters. During process validation, you must first address your special cause variation before you can address common cause variation. Otherwise, you introduce new problems.
Step 1: Remove Special Causes of Variation. Special causes of variation refer to assignable events, which can be either desirable or undesirable. Your body temperature, blood pressure and heart rate naturally fluctuate throughout the day without indicating any problems. If you get sick and have a fever, that would be a special cause of variation, and you might want to address that in some special way. If a special cause of variation is desirable, you want to understand and standardize. For undesirable special causes, you want to understand the cause and implement a countermeasure to reduce the risk of recurrence. Only after addressing special causes of variation can you achieve a stable process that is in control.
Step 2: Address Common Causes of Variation. Common causes of variation refer to natural variation which does not have an assignable cause. The weight of a bottle of water, the length of a pencil, the month’s sales will all vary on some level from one to another. This natural variation does not necessarily mean something bad happened. These seemingly random fluctuations occur on some level regardless of the controls you have in place. If common cause variations result in lower levels of accuracy or precision than are acceptable, you do not want to waste resources trying to find and address the cause. You do want to improve your process so that it can achieve higher levels of accuracy and/or precision.
If you treat special cause variation with common cause solutions or vice versa, you risk making things worse. You can end up over adjusting a stable process or exasperating an out-of-control process.
What Is Risk?
Process validation will also help reduce your manufacturing risk. You can think of risk as uncertainty. As the deviation from an expected outcome increases, risk increases.
Risk is different from quality. You could have low risk and low quality. This would mean you would be certain that you would have bad quality.
You achieve low risk in quality before, during, and after the manufacturing process:
- Designing. Before manufacture, quality by design focuses on improving the design of the part for better functionality, usability, manufacturability and serviceability.
- Processing. During manufacturing, you can monitor a quality process that produces quality parts.
- Inspecting. After manufacturing parts, you can inspect some or all of those units to know with a higher level of certainty that you have good products.
What Is a Quality Process?
A quality process is a capable, stable process that you know with certainty will produce conforming parts. Achieving quality through processing might be the least understood and most underutilized method, which is too bad because it provides a powerful tool for increasing quality and certainty. Achieving a quality process allows you to monitor your processing inputs and know with certainty what outputs you will get and that those outputs will conform to requirements.
A capable process will produce parts within the upper and lower specification limits. This tells you that your process makes parts that meet requirements. It does not tell you whether you are meeting requirements with certainty. You might be meeting requirements for now with a high risk of not meeting requirements at any point in the future.
The process is stable, or in control, once you have removed the special causes of variation. A stable process will produce parts within the upper and lower control limits. You monitor your process to ensure that the only variation your process experiences is common cause variation. If a new special cause of variation emerges, you want to address that and bring your process back into control.
A process can be capable but not stable. Perhaps the assignable causes of variation do not push your manufactured parts out of specification. In this situation, you want to identify the special causes of variation and reduce their impact or remove them.
A process can also be stable but not capable if it is in control but happens to reside outside the upper and lower specification limits. In this case, you want to adjust the process or improve the process in some way. You first address the special causes of variation to then validate your process.
How Do You Validate Your Process for Quality Parts?
When you qualify a process, you define the processing window that you know will create quality parts. This gives you validation of your process, which is different from validation of your product. Validation of your process confirms your process creates the part within specifications with consistency. Validation of your product tells you that your product works as intended.
Before starting qualification of your process, you need to make sure you have laid the groundwork. You want to define quality for your product. This includes identifying the critical attributes and their tolerances. Once you have this, you are ready to follow the three steps for process validation.
Process Validation Step 1: Conformance – Characterize your Process
An initial characterization experiment adjusts various input variables to analyze their level of impact on the critical attributes. Those critical process parameters might be some variation of temperature, pressure, time and speed.
With this design of experiments, you can characterize a process that creates your product within specification. You want to know that the variation of your output will still fall within specifications. To do this you want to optimize your process by adjusting the combinations of your input variables in a factorial experiment that leads to outputs that fall within your specifications. This gives you a process where you can inspect the resulting parts and conclude based on the variation of the attributes that you can deliver conforming parts with consistency (see Figure 2).
Remember that your process must be free from special causes of variation. Also, understand that this only addresses the variation of your output at that one process. It does not account for variation in the input parameters of the process.
Process Validation Step 2: Capability – Qualify your Process Windows
In reality, the input parameters of your process will vary due to common cause variation. Temperature and humidity can fluctuate with the weather. One operator will do things slightly different from another. If you leave yourself vulnerable to these fluctuations, you could end up with nonconforming product and little clue as to what caused the nonconformity.
This can happen when the nominal process being used resides on the edge of the allowable process window. For this reason, you want to push the limits of your process to see at what point the combinations of your input parameters cause nonconformities.
With a full process qualification, you start with your aesthetic process window, which gives you parts that look good. Within that window you find your dimensional process window, which gives you parts that conform dimensionally. Then you find an even smaller window, which represents the processing window which gives you parts on an ongoing basis that conform to requirements (see Figure 3). With this information, you can select the parameters for a nominal process that resides safely within your processing window. This way when your parameters experience variation, your process still produces quality parts.
Process Validation Step 3: Stability – Control your Process
At this point you want to monitor your process. You can do this by monitoring either your output through product inspection or your input parameters. If your input parameters remain within your established processing window you can have a high degree of confidence that your process produces good parts. Your process is stable if it is in statistical process control. This means you are not experiencing special cause variations in your input parameters or in the quality of your outputs (see Figure 4).
Conclusion
You want both process capability and process stability in order to know your quality is high and your risk is low. You can achieve this by following steps to validate your manufacturing process for conformance, capability and stability. Then you can know with confidence that your process will make quality parts with consistency.